Stochastic nanoroughness modulates neuron-astrocyte interactions and function via mechanosensing cation channels
- PMID: 25349433
- PMCID: PMC4234571
- DOI: 10.1073/pnas.1412740111
Stochastic nanoroughness modulates neuron-astrocyte interactions and function via mechanosensing cation channels
Abstract
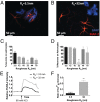
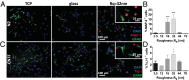
Extracellular soluble signals are known to play a critical role in maintaining neuronal function and homeostasis in the CNS. However, the CNS is also composed of extracellular matrix macromolecules and glia support cells, and the contribution of the physical attributes of these components in maintenance and regulation of neuronal function is not well understood. Because these components possess well-defined topography, we theorize a role for topography in neuronal development and we demonstrate that survival and function of hippocampal neurons and differentiation of telencephalic neural stem cells is modulated by nanoroughness. At roughnesses corresponding to that of healthy astrocytes, hippocampal neurons dissociated and survived independent from astrocytes and showed superior functional traits (increased polarity and calcium flux). Furthermore, telencephalic neural stem cells differentiated into neurons even under exogenous signals that favor astrocytic differentiation. The decoupling of neurons from astrocytes seemed to be triggered by changes to astrocyte apical-surface topography in response to nanoroughness. Blocking signaling through mechanosensing cation channels using GsMTx4 negated the ability of neurons to sense the nanoroughness and promoted decoupling of neurons from astrocytes, thus providing direct evidence for the role of nanotopography in neuron-astrocyte interactions. We extrapolate the role of topography to neurodegenerative conditions and show that regions of amyloid plaque buildup in brain tissue of Alzheimer's patients are accompanied by detrimental changes in tissue roughness. These findings suggest a role for astrocyte and ECM-induced topographical changes in neuronal pathologies and provide new insights for developing therapeutic targets and engineering of neural biomaterials.
Keywords: FAM38A; Piezo-1; mechanotransduction; polarization; stretch-activated channels.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Steindler DA, Pincus DW. Stem cells and neuropoiesis in the adult human brain. Lancet. 2002;359(9311):1047–1054. - PubMed
-
- Dessaud E, et al. Interpretation of the sonic hedgehog morphogen gradient by a temporal adaptation mechanism. Nature. 2007;450(7170):717–720. - PubMed
-
- Deverman BE, Patterson PH. Cytokines and CNS development. Neuron. 2009;64(1):61–78. - PubMed
-
- Rowitch DH, Kriegstein AR. Developmental genetics of vertebrate glial-cell specification. Nature. 2010;468(7321):214–222. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

