Developmental plasticity, cell fate specification and morphogenesis in the early mouse embryo
- PMID: 25349447
- PMCID: PMC4216461
- DOI: 10.1098/rstb.2013.0538
Developmental plasticity, cell fate specification and morphogenesis in the early mouse embryo
Erratum in
-
Correction to: 'Developmental plasticity, cell fate specification and morphogenesis in the early mouse embryo'.Philos Trans R Soc Lond B Biol Sci. 2015 Feb 5;370(1661):20140339. doi: 10.1098/rstb.2014.0339. Philos Trans R Soc Lond B Biol Sci. 2015. PMID: 25533108 Free PMC article. No abstract available.
Abstract
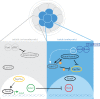
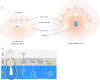
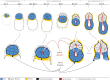
A critical point in mammalian development is when the early embryo implants into its mother's uterus. This event has historically been difficult to study due to the fact that it occurs within the maternal tissue and therefore is hidden from view. In this review, we discuss how the mouse embryo is prepared for implantation and the molecular mechanisms involved in directing and coordinating this crucial event. Prior to implantation, the cells of the embryo are specified as precursors of future embryonic and extra-embryonic lineages. These preimplantation cell fate decisions rely on a combination of factors including cell polarity, position and cell-cell signalling and are influenced by the heterogeneity between early embryo cells. At the point of implantation, signalling events between the embryo and mother, and between the embryonic and extraembryonic compartments of the embryo itself, orchestrate a total reorganization of the embryo, coupled with a burst of cell proliferation. New developments in embryo culture and imaging techniques have recently revealed the growth and morphogenesis of the embryo at the time of implantation, leading to a new model for the blastocyst to egg cylinder transition. In this model, pluripotent cells that will give rise to the fetus self-organize into a polarized three-dimensional rosette-like structure that initiates egg cylinder formation.
Keywords: cell fate; differentiation; embryo; morphogenesis; pluripotency.
Figures


References
-
- Suwińska A, Czołowska R, Ożdżeński W, Tarkowski AK. 2008. Blastomeres of the mouse embryo lose totipotency after the fifth cleavage division: expression of Cdx2 and Oct4 and developmental potential of inner and outer blastomeres of 16- and 32-cell embryos. Dev. Biol. 322, 133–144. (10.1016/j.ydbio.2008.07.019) - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

