Quercetin-3-glucoside increases low-density lipoprotein receptor (LDLR) expression, attenuates proprotein convertase subtilisin/kexin 9 (PCSK9) secretion, and stimulates LDL uptake by Huh7 human hepatocytes in culture
- PMID: 25349780
- PMCID: PMC4208090
- DOI: 10.1016/j.fob.2014.08.003
Quercetin-3-glucoside increases low-density lipoprotein receptor (LDLR) expression, attenuates proprotein convertase subtilisin/kexin 9 (PCSK9) secretion, and stimulates LDL uptake by Huh7 human hepatocytes in culture
Abstract
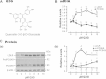
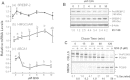
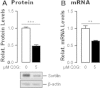
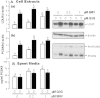
Low-density lipoprotein receptor (LDLR) mediates hepatic clearance of plasma cholesterol; proprotein convertase subtilisin/kexin 9 (PCSK9) opposes this clearance by promoting LDLR degradation. The plant flavonoid quercetin-3-β-d-glucoside (Q3G) has been shown to reduce hypercholesterolemia in experimental animals. Here, we examined how it affects LDLR and PCSK9 expression as well as LDL uptake by human Huh7 hepatocytes. At low micromolar concentrations, Q3G increased LDLR expression, reduced PCSK9 secretion, and stimulated LDL uptake. It also diminished intracellular sortilin, a sorting receptor known to facilitate PCSK9 secretion. Thus, as an LDLR inducer and a PCSK9 anti-secretagogue, Q3G may represent an effective anti-cholesterolemic agent.
Keywords: ABCA1, ATP-binding cassette transporter A1; ApoB, apolipoprotein B; Cholesterol; HMGCoAR, 3-hydroxy-3-methylglutaryl-CoA reductase; Hepatocytes; LDL-C, low-density lipoprotein-cholesterol; LDLR, low-density lipoprotein receptor; Low-density lipoprotein receptor; PCSK9, proprotein convertase subtilisin/kexin 9; Proprotein convertase; Q3G, quercetin-3-β-d-glucoside; Quercetin; SREBP-2, sterol regulatory element-binding protein 2; Sortilin; TGN, trans Golgi network.
Figures

Similar articles
-
Mice Fed a High-Cholesterol Diet Supplemented with Quercetin-3-Glucoside Show Attenuated Hyperlipidemia and Hyperinsulinemia Associated with Differential Regulation of PCSK9 and LDLR in their Liver and Pancreas.Mol Nutr Food Res. 2018 May;62(9):e1700729. doi: 10.1002/mnfr.201700729. Epub 2018 Apr 23. Mol Nutr Food Res. 2018. PMID: 29396908
-
Ascorbic acid enhances low-density lipoprotein receptor expression by suppressing proprotein convertase subtilisin/kexin 9 expression.J Biol Chem. 2020 Nov 20;295(47):15870-15882. doi: 10.1074/jbc.RA120.015623. Epub 2020 Sep 10. J Biol Chem. 2020. PMID: 32913121 Free PMC article.
-
Targeting the proprotein convertase subtilisin/kexin type 9 for the treatment of dyslipidemia and atherosclerosis.J Am Coll Cardiol. 2013 Oct 15;62(16):1401-8. doi: 10.1016/j.jacc.2013.07.056. Epub 2013 Aug 21. J Am Coll Cardiol. 2013. PMID: 23973703 Review.
-
Cyclase-associated protein 1 is a binding partner of proprotein convertase subtilisin/kexin type-9 and is required for the degradation of low-density lipoprotein receptors by proprotein convertase subtilisin/kexin type-9.Eur Heart J. 2020 Jan 7;41(2):239-252. doi: 10.1093/eurheartj/ehz566. Eur Heart J. 2020. PMID: 31419281 Free PMC article.
-
Proprotein Convertase Subtilisin/Kexin-Type 9 and Lipid Metabolism.Adv Exp Med Biol. 2020;1276:137-156. doi: 10.1007/978-981-15-6082-8_9. Adv Exp Med Biol. 2020. PMID: 32705598 Review.
Cited by
-
Hypocholesterolemic effect of quercetin-rich onion peel extract in C57BL/6J mice fed with high cholesterol diet.Food Sci Biotechnol. 2016 Jun 30;25(3):855-860. doi: 10.1007/s10068-016-0141-4. eCollection 2016. Food Sci Biotechnol. 2016. PMID: 30263345 Free PMC article.
-
Effects of alfalfa saponin extract on mRNA expression of Ldlr, LXRα, and FXR in BRL cells.J Zhejiang Univ Sci B. 2015 Jun;16(6):479-86. doi: 10.1631/jzus.B1400343. J Zhejiang Univ Sci B. 2015. PMID: 26055909 Free PMC article.
-
A Novel Promising Frontier for Human Health: The Beneficial Effects of Nutraceuticals in Cardiovascular Diseases.Int J Mol Sci. 2020 Nov 18;21(22):8706. doi: 10.3390/ijms21228706. Int J Mol Sci. 2020. PMID: 33218062 Free PMC article. Review.
-
Quercetin protects against ox‑LDL‑induced injury via regulation of ABCAl, LXR‑α and PCSK9 in RAW264.7 macrophages.Mol Med Rep. 2018 Jul;18(1):799-806. doi: 10.3892/mmr.2018.9048. Epub 2018 May 22. Mol Med Rep. 2018. PMID: 29845234 Free PMC article.
-
Non-antibody Approaches to Proprotein Convertase Subtilisin Kexin 9 Inhibition: siRNA, Antisense Oligonucleotides, Adnectins, Vaccination, and New Attempts at Small-Molecule Inhibitors Based on New Discoveries.Front Cardiovasc Med. 2019 Jan 29;5:199. doi: 10.3389/fcvm.2018.00199. eCollection 2018. Front Cardiovasc Med. 2019. PMID: 30761308 Free PMC article. Review.
References
-
- Bittner V. Non-high-density lipoprotein cholesterol and cardiovascular disease. Curr. Opin. Lipidol. 2003;14(4):367–371. - PubMed
-
- Mbikay M., Mayne J., Chretien M. Proprotein convertases subtilisin/kexin type 9, an enzyme turned escort protein: hepatic and extra hepatic functions. J. Diabetes. 2013;5(4):391–405. - PubMed
-
- Seidah N.G. The proprotein convertases, 20 years later. Methods Mol. Biol. 2011;768:23–57. - PubMed
-
- Chretien M. My road to Damascus: how I converted to the prohormone theory and the proprotein convertases. Biochem. Cell Biol. 2012;90(6):750–768. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

