Equine model for soft-tissue regeneration
- PMID: 25350377
- PMCID: PMC4868549
- DOI: 10.1002/jbm.b.33299
Equine model for soft-tissue regeneration
Abstract
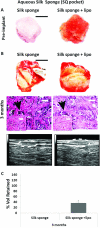
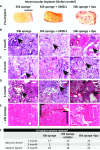
Soft-tissue regeneration methods currently yield suboptimal clinical outcomes due to loss of tissue volume and a lack of functional tissue regeneration. Grafted tissues and natural biomaterials often degrade or resorb too quickly, while most synthetic materials do not degrade. In previous research we demonstrated that soft-tissue regeneration can be supported using silk porous biomaterials for at least 18 months in vivo in a rodent model. In the present study, we scaled the system to a survival study using a large animal model and demonstrated the feasibility of these biomaterials for soft-tissue regeneration in adult horses. Both slow and rapidly degrading silk matrices were evaluated in subcutaneous pocket and intramuscular defect depots. We showed that we can effectively employ an equine model over 6 months to simultaneously evaluate many different implants, reducing the number of animals needed. Furthermore, we were able to tailor matrix degradation by varying the initial format of the implanted silk. Finally, we demonstrate ultrasound imaging of implants to be an effective means for tracking tissue regeneration and implant degradation.
Keywords: animal model; in vivo test; mesenchymal stem cell; scaffold; silk.
© 2014 Wiley Periodicals, Inc.
Figures


References
-
- Corona BT, Machingal MA, Criswell T, Vadhavkar M, Dannahower AC, Bergman C, et al. Further development of a tissue engineered muscle repair construct in vitro for enhanced functional recovery following implantation in vivo in a murine model of volumetric muscle loss injury. Tissue Eng Part A. 2012;18:1213–28. - PMC - PubMed
-
- Patrick C. Tissue engineering strategies for adipose tissue repair. Anat Rec. 2001;366:361–6. - PubMed
-
- De Sousa A. Psychological issues in oral and maxillofacial reconstructive surgery. Br J Oral Maxillofac Surg. 2008;46:661–4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

