Sustained radiosensitization of hypoxic glioma cells after oxygen pretreatment in an animal model of glioblastoma and in vitro models of tumor hypoxia
- PMID: 25350400
- PMCID: PMC4211739
- DOI: 10.1371/journal.pone.0111199
Sustained radiosensitization of hypoxic glioma cells after oxygen pretreatment in an animal model of glioblastoma and in vitro models of tumor hypoxia
Abstract
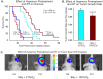
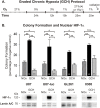
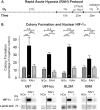
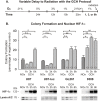
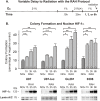
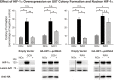
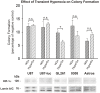
Glioblastoma multiforme (GBM) is the most common and lethal form of brain cancer and these tumors are highly resistant to chemo- and radiotherapy. Radioresistance is thought to result from a paucity of molecular oxygen in hypoxic tumor regions, resulting in reduced DNA damage and enhanced cellular defense mechanisms. Efforts to counteract tumor hypoxia during radiotherapy are limited by an attendant increase in the sensitivity of healthy brain tissue to radiation. However, the presence of heightened levels of molecular oxygen during radiotherapy, while conventionally deemed critical for adjuvant oxygen therapy to sensitize hypoxic tumor tissue, might not actually be necessary. We evaluated the concept that pre-treating tumor tissue by transiently elevating tissue oxygenation prior to radiation exposure could increase the efficacy of radiotherapy, even when radiotherapy is administered after the return of tumor tissue oxygen to hypoxic baseline levels. Using nude mice bearing intracranial U87-luciferase xenografts, and in vitro models of tumor hypoxia, the efficacy of oxygen pretreatment for producing radiosensitization was tested. Oxygen-induced radiosensitization of tumor tissue was observed in GBM xenografts, as seen by suppression of tumor growth and increased survival. Additionally, rodent and human glioma cells, and human glioma stem cells, exhibited prolonged enhanced vulnerability to radiation after oxygen pretreatment in vitro, even when radiation was delivered under hypoxic conditions. Over-expression of HIF-1α reduced this radiosensitization, indicating that this effect is mediated, in part, via a change in HIF-1-dependent mechanisms. Importantly, an identical duration of transient hyperoxic exposure does not sensitize normal human astrocytes to radiation in vitro. Taken together, these results indicate that briefly pre-treating tumors with elevated levels of oxygen prior to radiotherapy may represent a means for selectively targeting radiation-resistant hypoxic cancer cells, and could serve as a safe and effective adjuvant to radiation therapy for patients with GBM.
Conflict of interest statement
Figures

References
-
- Kanu OO, Mehta A, Di C, Lin N, Bortoff K, et al. (2009) Glioblastoma multiforme: a review of therapeutic targets. Expert Opin Ther Targets 13: 701–718. - PubMed
-
- Lakka SS, Gondi CS, Yanamandra N, Olivero WC, Dinh DH, et al. (2004) Inhibition of cathepsin B and MMP-9 gene expression in glioblastoma cell line via RNA interference reduces tumor cell invasion, tumor growth and angiogenesis. Oncogene 23: 4681–4689. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

