β2-Adrenergic receptor-dependent attenuation of hypoxic pulmonary vasoconstriction prevents progression of pulmonary arterial hypertension in intermittent hypoxic rats
- PMID: 25350545
- PMCID: PMC4211686
- DOI: 10.1371/journal.pone.0110693
β2-Adrenergic receptor-dependent attenuation of hypoxic pulmonary vasoconstriction prevents progression of pulmonary arterial hypertension in intermittent hypoxic rats
Abstract
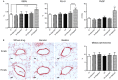
In sleep apnea syndrome (SAS), intermittent hypoxia (IH) induces repeated episodes of hypoxic pulmonary vasoconstriction (HPV) during sleep, which presumably contribute to pulmonary arterial hypertension (PAH). However, the prevalence of PAH was low and severity is mostly mild in SAS patients, and mild or no right ventricular hypertrophy (RVH) was reported in IH-exposed animals. The question then arises as to why PAH is not a universal finding in SAS if repeated hypoxia of sufficient duration causes cycling HPV. In the present study, rats underwent IH at a rate of 3 min cycles of 4-21% O2 for 8 h/d for 6 w. Assessment of diameter changes in small pulmonary arteries in response to acute hypoxia and drugs were performed using synchrotron radiation microangiography on anesthetized rats. In IH-rats, neither PAH nor RVH was observed and HPV was strongly reversed. Nadolol (a hydrophilic β(1, 2)-blocker) augmented the attenuated HPV to almost the same level as that in N-rats, but atenolol (a hydrophilic β1-blocker) had no effect on the HPV in IH. These β-blockers had almost no effect on the HPV in N-rats. Chronic administration of nadolol during 6 weeks of IH exposure induced PAH and RVH in IH-rats, but did not in N-rats. Meanwhile, atenolol had no effect on morphometric and hemodynamic changes in N and IH-rats. Protein expression of the β1-adrenergic receptor (AR) was down-regulated while that of β2AR was preserved in pulmonary arteries of IH-rats. Phosphorylation of p85 (chief component of phosphoinositide 3-kinase (PI3K)), protein kinase B (Akt), and endothelial nitric oxide synthase (eNOS) were abrogated by chronic administration of nadolol in the lung tissue of IH-rats. We conclude that IH-derived activation of β2AR in the pulmonary arteries attenuates the HPV, thereby preventing progression of IH-induced PAH. This protective effect may depend on the β2AR-Gi mediated PI3K/Akt/eNOS signaling pathway.
Conflict of interest statement
Figures




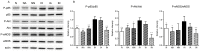
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

