Examination of the combined effects of chondroitinase ABC, growth factors and locomotor training following compressive spinal cord injury on neuroanatomical plasticity and kinematics
- PMID: 25350665
- PMCID: PMC4211738
- DOI: 10.1371/journal.pone.0111072
Examination of the combined effects of chondroitinase ABC, growth factors and locomotor training following compressive spinal cord injury on neuroanatomical plasticity and kinematics
Abstract
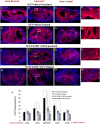
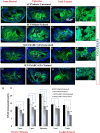
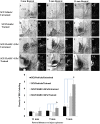
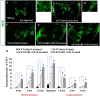
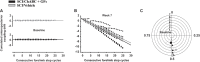
While several cellular and pharmacological treatments have been evaluated following spinal cord injury (SCI) in animal models, it is increasingly recognized that approaches to address the glial scar, including the use of chondroitinase ABC (ChABC), can facilitate neuroanatomical plasticity. Moreover, increasing evidence suggests that combinatorial strategies are key to unlocking the plasticity that is enabled by ChABC. Given this, we evaluated the anatomical and functional consequences of ChABC in a combinatorial approach that also included growth factor (EGF, FGF2 and PDGF-AA) treatments and daily treadmill training on the recovery of hindlimb locomotion in rats with mid thoracic clip compression SCI. Using quantitative neuroanatomical and kinematic assessments, we demonstrate that the combined therapy significantly enhanced the neuroanatomical plasticity of major descending spinal tracts such as corticospinal and serotonergic-spinal pathways. Additionally, the pharmacological treatment attenuated chronic astrogliosis and inflammation at and adjacent to the lesion with the modest synergistic effects of treadmill training. We also observed a trend for earlier recovery of locomotion accompanied by an improvement of the overall angular excursions in rats treated with ChABC and growth factors in the first 4 weeks after SCI. At the end of the 7-week recovery period, rats from all groups exhibited an impressive spontaneous recovery of the kinematic parameters during locomotion on treadmill. However, although the combinatorial treatment led to clear chronic neuroanatomical plasticity, these structural changes did not translate to an additional long-term improvement of locomotor parameters studied including hindlimb-forelimb coupling. These findings demonstrate the beneficial effects of combined ChABC, growth factors and locomotor training on the plasticity of the injured spinal cord and the potential to induce earlier neurobehavioral recovery. However, additional approaches such as stem cell therapies or a more adapted treadmill training protocol may be required to optimize this repair strategy in order to induce sustained functional locomotor improvement.
Conflict of interest statement
Figures




References
-
- Sekhon LH, Fehlings MG (2001) Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine 26: S2–12. - PubMed
-
- Tator CH (1998) Biology of Neurological Recovery and Functional Restoration after Spinal Cord Injury. Neurosurgery 42: 696–708. - PubMed
-
- van Hedel HJ, Dietz V (2010) Rehabilitation of locomotion after spinal cord injury. Restor Neurol Neurosci 28: 123–134. - PubMed
-
- Fawcett JW, Curt A, Steeves JD, Coleman WP, Tuszynski MH, et al. (2007) Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord 45: 190–205. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

