A pilot study to develop a diagnostic test for pancreatic ductal adenocarcinoma based on differential expression of select miRNA in plasma and bile
- PMID: 25350767
- PMCID: PMC4261139
- DOI: 10.1038/ajg.2014.331
A pilot study to develop a diagnostic test for pancreatic ductal adenocarcinoma based on differential expression of select miRNA in plasma and bile
Abstract
Objectives: Accurate peripheral markers for the diagnosis of pancreatic ductal adenocarcinoma (PDAC) are lacking. We measured the differential expression of select microRNAs (miRNAs) in plasma and bile among patients with PDAC, chronic pancreatitis (CP), and controls.
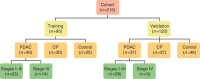
Methods: We identified patients (n=215) with treatment-naive PDAC (n=77), CP with bile/pancreatic duct pathology (n=67), and controls (n=71) who had been prospectively enrolled in a Pancreatobiliary Biorepository at the time of endoscopic retrograde cholangiopancreatography or endoscopic ultrasound. Controls were patients with choledocholithiasis but normal pancreata. The sample was separated into training (n=95) and validation (n=120) cohorts to establish and then test the performance of PDAC Signature Panels in diagnosing PDAC. The training cohort (n=95) included age-matched patients with PDAC, CP, and controls. Panels were derived from the differential expression of 10 candidate miRNAs in plasma or bile. We selected miRNAs having excellent accuracy for inclusion in regression models.
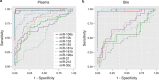
Results: Using the training cohort, we confirmed the differential expression of 9/10 miRNAs in plasma (miR-10b, -30c, -106b, -132, -155, -181a, -181b, -196a, and -212) and 7/10 in bile (excluding miR-21, -132, and -181b). Of these, five (miR-10b, -155, -106b, -30c, and -212) had excellent accuracy for distinguishing PDAC. In the training and validation cohorts, the sensitivity/specificity for a PDAC Panel derived from plasma was 95/100% and 100/100%, respectively; in bile, these were 96/100% and 100/100%.
Conclusions: Increased expression of miRNA-10b, -155, and -106b in plasma appears highly accurate in diagnosing PDAC. Additional studies are needed to confirm this Panel and explore its value as a prognostic test.
Figures


Comment in
-
Usefulness of Measuring microRNAs in Bile and Plasma for Pancreatic Ductal Adenocarcinoma Diagnosis.Am J Gastroenterol. 2015 May;110(5):768-9. doi: 10.1038/ajg.2015.77. Am J Gastroenterol. 2015. PMID: 25942302 No abstract available.
-
Response to Le Large et al.Am J Gastroenterol. 2015 May;110(5):769-70. doi: 10.1038/ajg.2015.82. Am J Gastroenterol. 2015. PMID: 25942303 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

