An inside-out origin for the eukaryotic cell
- PMID: 25350791
- PMCID: PMC4210606
- DOI: 10.1186/s12915-014-0076-2
An inside-out origin for the eukaryotic cell
Abstract
Background: Although the origin of the eukaryotic cell has long been recognized as the single most profound change in cellular organization during the evolution of life on earth, this transition remains poorly understood. Models have always assumed that the nucleus and endomembrane system evolved within the cytoplasm of a prokaryotic cell.
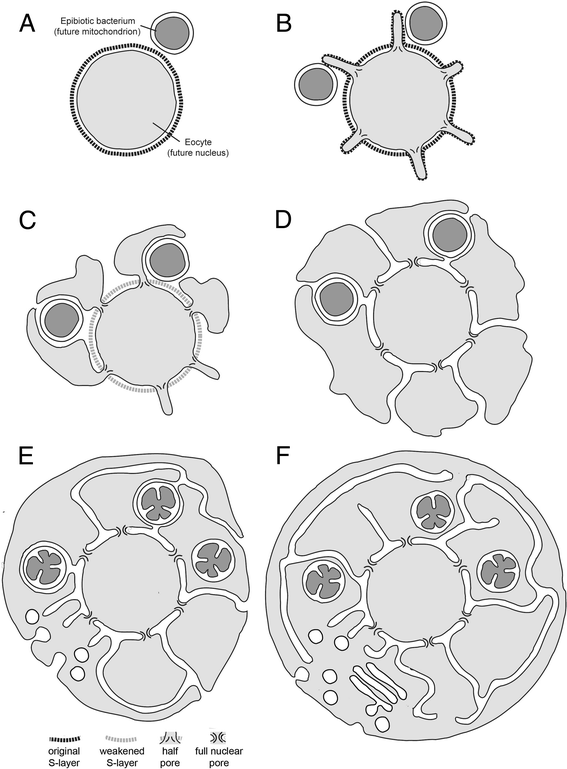
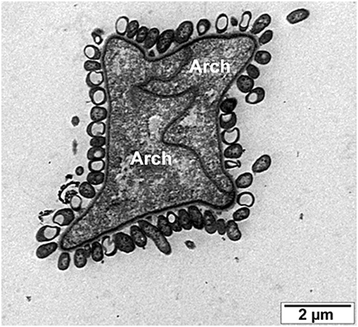
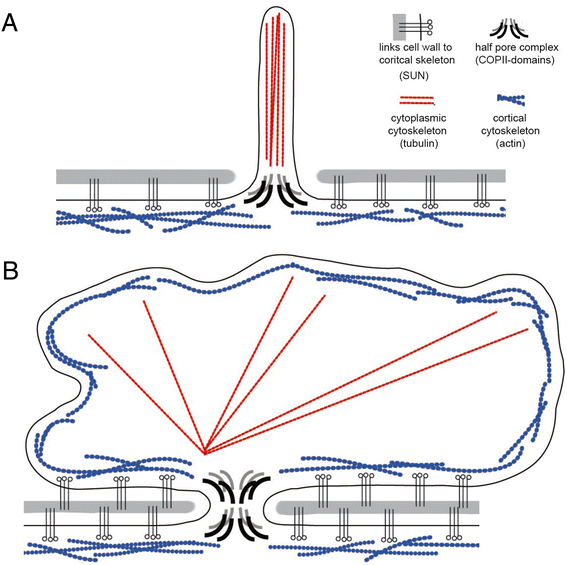
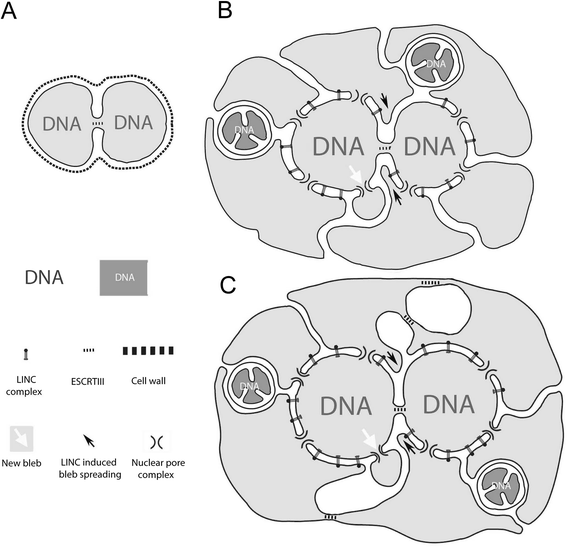
Results: Drawing on diverse aspects of cell biology and phylogenetic data, we invert the traditional interpretation of eukaryotic cell evolution. We propose that an ancestral prokaryotic cell, homologous to the modern-day nucleus, extruded membrane-bound blebs beyond its cell wall. These blebs functioned to facilitate material exchange with ectosymbiotic proto-mitochondria. The cytoplasm was then formed through the expansion of blebs around proto-mitochondria, with continuous spaces between the blebs giving rise to the endoplasmic reticulum, which later evolved into the eukaryotic secretory system. Further bleb-fusion steps yielded a continuous plasma membrane, which served to isolate the endoplasmic reticulum from the environment.
Conclusions: The inside-out theory is consistent with diverse kinds of data and provides an alternative framework by which to explore and understand the dynamic organization of modern eukaryotic cells. It also helps to explain a number of previously enigmatic features of cell biology, including the autonomy of nuclei in syncytia and the subcellular localization of protein N-glycosylation, and makes many predictions, including a novel mechanism of interphase nuclear pore insertion.
Figures



References
-
- Margulis L. Origin of Eukaryotic Cells. New Haven: Yale University Press; 1970.
-
- Margulis L. Symbiosis in Cell Evolution. New York: W. H. Freeman; 1981.
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

