IL-33-dependent type 2 inflammation during rhinovirus-induced asthma exacerbations in vivo
- PMID: 25350863
- PMCID: PMC4299647
- DOI: 10.1164/rccm.201406-1039OC
IL-33-dependent type 2 inflammation during rhinovirus-induced asthma exacerbations in vivo
Abstract
Rationale: Rhinoviruses are the major cause of asthma exacerbations; however, its underlying mechanisms are poorly understood. We hypothesized that the epithelial cell-derived cytokine IL-33 plays a central role in exacerbation pathogenesis through augmentation of type 2 inflammation.
Objectives: To assess whether rhinovirus induces a type 2 inflammatory response in asthma in vivo and to define a role for IL-33 in this pathway.
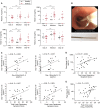
Methods: We used a human experimental model of rhinovirus infection and novel airway sampling techniques to measure IL-4, IL-5, IL-13, and IL-33 levels in the asthmatic and healthy airways during a rhinovirus infection. Additionally, we cultured human T cells and type 2 innate lymphoid cells (ILC2s) with the supernatants of rhinovirus-infected bronchial epithelial cells (BECs) to assess type 2 cytokine production in the presence or absence of IL-33 receptor blockade.
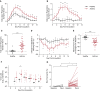
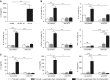
Measurements and main results: IL-4, IL-5, IL-13, and IL-33 are all induced by rhinovirus in the asthmatic airway in vivo and relate to exacerbation severity. Further, induction of IL-33 correlates with viral load and IL-5 and IL-13 levels. Rhinovirus infection of human primary BECs induced IL-33, and culture of human T cells and ILC2s with supernatants of rhinovirus-infected BECs strongly induced type 2 cytokines. This induction was entirely dependent on IL-33.
Conclusions: IL-33 and type 2 cytokines are induced during a rhinovirus-induced asthma exacerbation in vivo. Virus-induced IL-33 and IL-33-responsive T cells and ILC2s are key mechanistic links between viral infection and exacerbation of asthma. IL-33 inhibition is a novel therapeutic approach for asthma exacerbations.
Keywords: ILC2; Th2; infection; virus.
Figures

Comment in
-
Interleukin-33: a potential link between rhinovirus infections and asthma exacerbation.Am J Respir Crit Care Med. 2014 Dec 15;190(12):1336-7. doi: 10.1164/rccm.201411-1949ED. Am J Respir Crit Care Med. 2014. PMID: 25496100 Free PMC article. No abstract available.
-
IL-33-dependent Th2 response after rhinovirus infection in asthma: more information needed.Am J Respir Crit Care Med. 2015 Jan 15;191(2):237. doi: 10.1164/rccm.201411-2067LE. Am J Respir Crit Care Med. 2015. PMID: 25590160 No abstract available.
-
IL-33-dependent type 2 inflammation in asthma exacerbations.Am J Respir Crit Care Med. 2015 Jan 15;191(2):237-8. doi: 10.1164/rccm.201411-2042LE. Am J Respir Crit Care Med. 2015. PMID: 25590161 No abstract available.
-
Reply: IL-33-dependent type 2 inflammation in asthma exacerbations.Am J Respir Crit Care Med. 2015 Jan 15;191(2):238. doi: 10.1164/rccm.201412-2134LE. Am J Respir Crit Care Med. 2015. PMID: 25590162 No abstract available.
References
-
- Grissell TV, Powell H, Shafren DR, Boyle MJ, Hensley MJ, Jones PD, Whitehead BF, Gibson PG. Interleukin-10 gene expression in acute virus-induced asthma. Am J Respir Crit Care Med. 2005;172:433–439. - PubMed
-
- Wenzel S, Wilbraham D, Fuller R, Getz EB, Longphre M. Effect of an interleukin-4 variant on late phase asthmatic response to allergen challenge in asthmatic patients: results of two phase 2a studies. Lancet. 2007;370:1422–1431. - PubMed
-
- Nair P, Pizzichini MMM, Kjarsgaard M, Inman MD, Efthimiadis A, Pizzichini E, Hargreave FE, O’Byrne PM. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl J Med. 2009;360:985–993. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

