Randomized withdrawal study of patients with symptomatic neurogenic orthostatic hypotension responsive to droxidopa
- PMID: 25350981
- PMCID: PMC4354798
- DOI: 10.1161/HYPERTENSIONAHA.114.04035
Randomized withdrawal study of patients with symptomatic neurogenic orthostatic hypotension responsive to droxidopa
Abstract
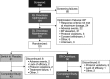
We evaluated whether droxidopa, a prodrug converted to norepinephrine, is beneficial in the treatment of symptomatic neurogenic orthostatic hypotension, which results from failure to generate an appropriate norepinephrine response to postural challenge. Patients with symptomatic neurogenic orthostatic hypotension and Parkinson disease, multiple system atrophy, pure autonomic failure, or nondiabetic autonomic neuropathy underwent open-label droxidopa titration (100-600 mg, 3× daily). Responders then received an additional 7-day open-label treatment at their individualized dose. Patients were subsequently randomized to continue with droxidopa or withdraw to placebo for 14 days. We then assessed patient-reported scores on the Orthostatic Hypotension Questionnaire and blood pressure measurements. Mean worsening of Orthostatic Hypotension Questionnaire dizziness/lightheadedness score from randomization to end of study (the primary outcome; N=101) was 1.9±3.2 with placebo and 1.3±2.8 units with droxidopa (P=0.509). Four of the other 5 Orthostatic Hypotension Questionnaire symptom scores and all 4 symptom-impact scores favored droxidopa, with statistical significance for the patient's self-reported ability to perform activities requiring standing a short time (P=0.033) and standing a long time (P=0.028). Furthermore, a post hoc analysis of a predefined composite score of all symptoms (Orthostatic Hypotension Questionnaire composite) demonstrated a significant benefit for droxidopa (P=0.013). There was no significant difference between groups for standing systolic blood pressure (P=0.680). Droxidopa was well tolerated. In summary, this randomized withdrawal droxidopa study failed to meet its primary efficacy end point. Additional clinical trials are needed to confirm that droxidopa is beneficial in symptomatic neurogenic orthostatic hypotension, as suggested by the positive secondary outcomes of this trial.
Clinical trial registration url: http://www.clinicaltrials.gov. Unique identifier: NCT00633880.
Keywords: Parkinson disease; autonomic nervous system; droxidopa; multiple system atrophy; norepinephrine.
© 2014 The Authors. Published on behalf of the American Heart Association, Inc., by Wolters Kluwer.
Figures
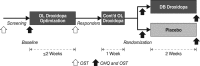


Comment in
-
Prospects for droxidopa in neurogenic orthostatic hypotension.Hypertension. 2015 Jan;65(1):34-5. doi: 10.1161/HYPERTENSIONAHA.114.04204. Epub 2014 Oct 27. Hypertension. 2015. PMID: 25350983 Free PMC article. No abstract available.
References
-
- Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. 2011;21:69–72. - PubMed
-
- Gangavati A, Hajjar I, Quach L, Jones RN, Kiely DK, Gagnon P, Lipsitz LA. Hypertension, orthostatic hypotension, and the risk of falls in a community-dwelling elderly population: the maintenance of balance, independent living, intellect, and zest in the elderly of Boston study. J Am Geriatr Soc. 2011;59:383–389. - PMC - PubMed
-
- Freeman R. Clinical practice. Neurogenic orthostatic hypotension. N Engl J Med. 2008;358:615–624. - PubMed
-
- Ooi WL, Hossain M, Lipsitz LA. The association between orthostatic hypotension and recurrent falls in nursing home residents. Am J Med. 2000;108:106–111. - PubMed
-
- Luukinen H, Koski K, Laippala P, Kivelä SL. Prognosis of diastolic and systolic orthostatic hypotension in older persons. Arch Intern Med. 1999;159:273–280. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

