Hypoxia-mediated downregulation of miRNA biogenesis promotes tumour progression
- PMID: 25351346
- PMCID: PMC4215647
- DOI: 10.1038/ncomms6202
Hypoxia-mediated downregulation of miRNA biogenesis promotes tumour progression
Erratum in
-
Author Correction: Hypoxia-mediated downregulation of miRNA biogenesis promotes tumour progression.Nat Commun. 2020 Jun 3;11(1):2867. doi: 10.1038/s41467-020-16661-3. Nat Commun. 2020. PMID: 32493919 Free PMC article.
Abstract
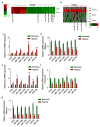
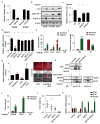
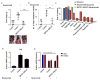
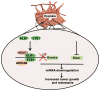
Cancer-related deregulation of miRNA biogenesis has been suggested, but the underlying mechanisms remain elusive. Here we report a previously unrecognized effect of hypoxia in the downregulation of Drosha and Dicer in cancer cells that leads to dysregulation of miRNA biogenesis and increased tumour progression. We show that hypoxia-mediated downregulation of Drosha is dependent on ETS1/ELK1 transcription factors. Moreover, mature miRNA array and deep sequencing studies reveal altered miRNA maturation in cells under hypoxic conditions. At a functional level, this phenomenon results in increased cancer progression in vitro and in vivo, and data from patient samples are suggestive of miRNA biogenesis downregulation in hypoxic tumours. Rescue of Drosha by siRNAs targeting ETS1/ELK1 in vivo results in significant tumour regression. These findings provide a new link in the mechanistic understanding of global miRNA downregulation in the tumour microenvironment.
Conflict of interest statement
Figures



References
-
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 CA016672/CA/NCI NIH HHS/United States
- U01 CA213759/CA/NCI NIH HHS/United States
- R01 CA109298/CA/NCI NIH HHS/United States
- CA016672/CA/NCI NIH HHS/United States
- 201592/CAPMC/ CIHR/Canada
- U24CA143835/CA/NCI NIH HHS/United States
- R01 CA155332/CA/NCI NIH HHS/United States
- T32 CA009666/CA/NCI NIH HHS/United States
- T32 CA101642/CA/NCI NIH HHS/United States
- CA109298/CA/NCI NIH HHS/United States
- UH2TR000943/TR/NCATS NIH HHS/United States
- U24 CA143835/CA/NCI NIH HHS/United States
- UH2 TR000943/TR/NCATS NIH HHS/United States
- R01 CA177909/CA/NCI NIH HHS/United States
- P50 CA098258/CA/NCI NIH HHS/United States
- CA177909/CA/NCI NIH HHS/United States
- U54 CA151668/CA/NCI NIH HHS/United States
- UL1 TR001108/TR/NCATS NIH HHS/United States
- P50 CA083639/CA/NCI NIH HHS/United States
- CA155332/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

