MicroRNA induced cardiac reprogramming in vivo: evidence for mature cardiac myocytes and improved cardiac function
- PMID: 25351576
- PMCID: PMC4312531
- DOI: 10.1161/CIRCRESAHA.116.304510
MicroRNA induced cardiac reprogramming in vivo: evidence for mature cardiac myocytes and improved cardiac function
Abstract
Rationale: A major goal for the treatment of heart tissue damaged by cardiac injury is to develop strategies for restoring healthy heart muscle through the regeneration and repair of damaged myocardium. We recently demonstrated that administration of a specific combination of microRNAs (miR combo) into the infarcted myocardium leads to direct in vivo reprogramming of noncardiac myocytes to cardiac myocytes. However, the biological and functional consequences of such reprogramming are not yet known.
Objective: The aim of this study was to determine whether noncardiac myocytes directly reprogrammed using miRNAs in vivo develop into mature functional cardiac myocytes in situ, and whether reprogramming leads to improvement of cardiac function.
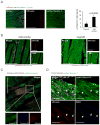
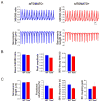
Methods and results: We subjected fibroblast-specific protein 1-Cre mice/tandem dimer Tomato (tdTomato) mice to cardiac injury by permanent ligation of the left anterior descending coronary artery and injected lentiviruses encoding miR combo or a control nontargeting miRNA. miR combo significantly increased the number of reprogramming events in vivo. Five to 6 weeks after injury, morphological and physiological properties of tdTomato(-) and tdTomato(+) cardiac myocyte-like cells were analyzed ex vivo. tdTomato(+) cells expressed cardiac myocyte markers, sarcomeric organization, excitation-contraction coupling, and action potentials characteristic of mature ventricular cardiac myocytes (tdTomato(-) cells). Reprogramming was associated with improvement of cardiac function, as analyzed by serial echocardiography. There was a time delayed and progressive improvement in fractional shortening and other measures of ventricular function, indicating that miR combo promotes functional recovery of damaged myocardium.
Conclusions: The findings from this study further validate the potential use of miRNA-mediated reprogramming as a therapeutic approach to promote cardiac regeneration after myocardial injury.
Keywords: cellular reprogramming; excitation–contraction coupling; guided tissue regeneration; microRNAs; myocardial infarction; myocytes, cardiac.
© 2014 American Heart Association, Inc.
Conflict of interest statement
None of the authors have any real or apparent conflict(s) of interest to disclose.
Figures



Comment in
-
Small RNAs make big impact in cardiac repair.Circ Res. 2015 Jan 30;116(3):393-5. doi: 10.1161/CIRCRESAHA.114.305676. Circ Res. 2015. PMID: 25634967 Free PMC article. No abstract available.
References
-
- Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd-Jones DM, Nelson SA, Nichol G, Orenstein D, Wilson PW, Woo YJ. Forecasting the future of cardiovascular disease in the united states: A policy statement from the american heart association. Circulation. 2011;123:933–944. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

