Cabozantinib overcomes crizotinib resistance in ROS1 fusion-positive cancer
- PMID: 25351743
- PMCID: PMC4286456
- DOI: 10.1158/1078-0432.CCR-14-1385
Cabozantinib overcomes crizotinib resistance in ROS1 fusion-positive cancer
Abstract
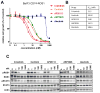
Purpose: ROS1 rearrangement leads to constitutive ROS1 activation with potent transforming activity. In an ongoing phase I trial, the ALK tyrosine kinase inhibitor (TKI) crizotinib shows remarkable initial responses in patients with non-small cell lung cancer (NSCLC) harboring ROS1 fusions; however, cancers eventually develop crizotinib resistance due to acquired mutations such as G2032R in ROS1. Thus, understanding the crizotinib-resistance mechanisms in ROS1-rearranged NSCLC and identification of therapeutic strategies to overcome the resistance are required.
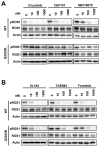
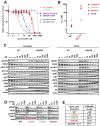
Experimental design: The sensitivity of CD74-ROS1-transformed Ba/F3 cells to multiple ALK inhibitors was examined. Acquired ROS1 inhibitor-resistant mutations in CD74-ROS1 fusion were screened by N-ethyl-N-nitrosourea mutagenesis with Ba/F3 cells. To overcome the resistance mutation, we performed high-throughput drug screening with small-molecular inhibitors and anticancer drugs used in clinical practice or being currently tested in clinical trials. The effect of the identified drug was assessed in the CD74-ROS1-mutant Ba/F3 cells and crizotinib-resistant patient-derived cancer cells (MGH047) harboring G2032R-mutated CD74-ROS1.
Results: We identified multiple novel crizotinib-resistance mutations in the ROS1 kinase domain, including the G2032R mutation. As the result of high-throughput drug screening, we found that the cMET/RET/VEGFR inhibitor cabozantinib (XL184) effectively inhibited the survival of CD74-ROS1 wild-type (WT) and resistant mutants harboring Ba/F3 and MGH047 cells. Furthermore, cabozantinib could overcome all the resistance by all newly identified secondary mutations.
Conclusions: We developed a comprehensive model of acquired resistance to ROS1 inhibitors in NSCLC with ROS1 rearrangement and identified cabozantinib as a therapeutic strategy to overcome the resistance.
©2014 American Association for Cancer Research.
Figures


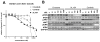
References
-
- Takeuchi K, Soda M, Togashi Y, Suzuki R, Sakata S, Hatano S, et al. RET, ROS1 and ALK fusions in lung cancer. Nat Med. 2012;18:378–81. - PubMed
-
- Choi YL, Soda M, Yamashita Y, Ueno T, Takashima J, Nakajima T, et al. EML4-ALK mutations in lung cancer that confer resistance to ALK inhibitors. N Engl J Med. 2010;363:1734–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

