Recent advances in primary ciliary dyskinesia genetics
- PMID: 25351953
- PMCID: PMC4285891
- DOI: 10.1136/jmedgenet-2014-102755
Recent advances in primary ciliary dyskinesia genetics
Abstract
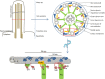
Primary ciliary dyskinesia (PCD) is a rare genetically heterogeneous disorder caused by the abnormal structure and/or function of motile cilia. The PCD diagnosis is challenging and requires a well-described clinical phenotype combined with the identification of abnormalities in ciliary ultrastructure and/or beating pattern as well as the recognition of genetic cause of the disease. Regarding the pace of identification of PCD-related genes, a rapid acceleration during the last 2-3 years is notable. This is the result of new technologies, such as whole-exome sequencing, that have been recently applied in genetic research. To date, PCD-causative mutations in 29 genes are known and the number of causative genes is bound to rise. Even though the genetic causes of approximately one-third of PCD cases still remain to be found, the current knowledge can already be used to create new, accurate genetic tests for PCD that can accelerate the correct diagnosis and reduce the proportion of unexplained cases. This review aims to present the latest data on the relations between ciliary structure aberrations and their genetic basis.
Keywords: Cell biology; Diagnostics; Genetics; Molecular genetics.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Figures

References
-
- Rompolas P, Pedersen L, Patel-King R, King S. Chlamydomonas FAP133 is a dynein intermediate chain associated with the retrograde intraflagellar transport motor. J Cell Sci 2007;120(Pt 20):3653–65. - PubMed
-
- Tissir F, Qu Y, Montcouquiol M, Zhou L, Komatsu K, Shi D, Fujimori T, Labeau J, Tyteca D, Courtoy P, Poumay Y, Uemura T, Goffinet AM. Lack of cadherins Celsr2 and Celsr3 impairs ependymal ciliogenesis, leading to fatal hydrocephalus. Nat Neurosci 2010;13:700–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
