The clinically approved drugs dasatinib and bosutinib induce anti-inflammatory macrophages by inhibiting the salt-inducible kinases
- PMID: 25351958
- PMCID: PMC4286194
- DOI: 10.1042/BJ20141165
The clinically approved drugs dasatinib and bosutinib induce anti-inflammatory macrophages by inhibiting the salt-inducible kinases
Abstract
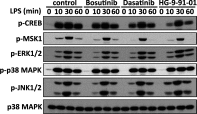
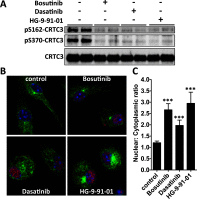
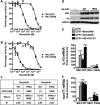
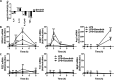
Macrophages switch to an anti-inflammatory, 'regulatory'-like phenotype characterized by the production of high levels of interleukin (IL)-10 and low levels of pro-inflammatory cytokines to promote the resolution of inflammation. A potential therapeutic strategy for the treatment of chronic inflammatory diseases would be to administer drugs that could induce the formation of 'regulatory'-like macrophages at sites of inflammation. In the present study, we demonstrate that the clinically approved cancer drugs bosutinib and dasatinib induce several hallmark features of 'regulatory'-like macrophages. Treatment of macrophages with bosutinib or dasatinib elevates the production of IL-10 while suppressing the production of IL-6, IL-12p40 and tumour necrosis factor α (TNFα) in response to Toll-like receptor (TLR) stimulation. Moreover, macrophages treated with bosutinib or dasatinib express higher levels of markers of 'regulatory'-like macrophages including LIGHT, SPHK1 and arginase 1. Bosutinib and dasatinib were originally developed as inhibitors of the protein tyrosine kinases Bcr-Abl and Src but we show that, surprisingly, the effects of bosutinib and dasatinib on macrophage polarization are the result of the inhibition of the salt-inducible kinases. Consistent with the present finding, bosutinib and dasatinib induce the dephosphorylation of CREB-regulated transcription co-activator 3 (CRTC3) and its nuclear translocation where it induces a cAMP-response-element-binding protein (CREB)-dependent gene transcription programme including that of IL-10. Importantly, these effects of bosutinib and dasatinib on IL-10 gene expression are lost in macrophages expressing a drug-resistant mutant of salt-inducible kinase 2 (SIK2). In conclusion, our study identifies the salt-inducible kinases as major targets of bosutinib and dasatinib that mediate the effects of these drugs on the innate immune system and provides novel mechanistic insights into the anti-inflammatory properties of these drugs.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

