Small heat-shock proteins: important players in regulating cellular proteostasis
- PMID: 25352169
- PMCID: PMC11113218
- DOI: 10.1007/s00018-014-1754-5
Small heat-shock proteins: important players in regulating cellular proteostasis
Abstract
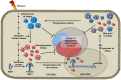
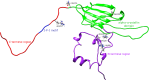
Small heat-shock proteins (sHsps) are a diverse family of intra-cellular molecular chaperone proteins that play a critical role in mitigating and preventing protein aggregation under stress conditions such as elevated temperature, oxidation and infection. In doing so, they assist in the maintenance of protein homeostasis (proteostasis) thereby avoiding the deleterious effects that result from loss of protein function and/or protein aggregation. The chaperone properties of sHsps are therefore employed extensively in many tissues to prevent the development of diseases associated with protein aggregation. Significant progress has been made of late in understanding the structure and chaperone mechanism of sHsps. In this review, we discuss some of these advances, with a focus on mammalian sHsp hetero-oligomerisation, the mechanism by which sHsps act as molecular chaperones to prevent both amorphous and fibrillar protein aggregation, and the role of post-translational modifications in sHsp chaperone function, particularly in the context of disease.
Figures


References
-
- Baldwin AJ, Knowles TP, Tartaglia GG, Fitzpatrick AW, Devlin GL, Shammas SL, Waudby CA, Mossuto MF, Meehan S, Gras SL, Christodoulou J, Anthony-Cahill SJ, Barker PD, Vendruscolo M, Dobson CM. Metastability of native proteins and the phenomenon of amyloid formation. J Am Chem Soc. 2011;133(36):14160–14163. - PubMed
-
- Hilton GR, Lioe H, Stengel F, Baldwin AJ, Benesch JLP. Small heat-shock proteins: paramedics of the cell. Top Curr Chem. 2013;328:69–98. - PubMed
-
- Laskowska E, Matuszewska E, Kuczynska-Wisnik D. Small heat-shock proteins and protein-misfolding diseases. Curr Pharm Biotechnol. 2010;11:146–157. - PubMed
-
- Mymrikov EV, Seit-Nebi AS, Gusev NB. Large potentials of small heat shock proteins. Physiol Rev. 2011;91:1123–1159. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

