Evolution of the Food and Drug Administration approach to liver safety assessment for new drugs: current status and challenges
- PMID: 25352324
- PMCID: PMC4212154
- DOI: 10.1007/s40264-014-0182-7
Evolution of the Food and Drug Administration approach to liver safety assessment for new drugs: current status and challenges
Abstract
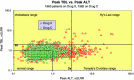
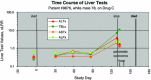
Prompted by approval in 1997 of troglitazone and bromfenac, two drugs that promptly began to show serious and sometimes fatal liver toxicity, we began at the Food and Drug Administration (FDA) a series of annual conferences in 1999 to consider issues of drug-induced liver injury (DILI). First inviting reviewers of new drug applications we opened the audiences in 2001 to pharmaceutical industry and academic consultants to industry and FDA, and slides shown at the meetings were posted on the internet to be available at the website of the American Association for the Study of Liver Diseases (AASLD)-go to ( http://www.aasld.org/dili/Pages/default.aspx ). Observations by Dr. Hyman J. Zimmerman that "drug-induced hepatocellular jaundice is a serious lesion" with possible mortality formed a basis for developing a computer program to plot peak serum values for alanine aminotransferase (ALT) and total bilirubin (TBL) in an x-y log-log graph for all subjects enrolled in clinical trials. This program had the capability to show the time course of all liver tests for individuals who had both hepatocellular injury and reduced whole liver function, plus clinical narratives to diagnose the severity and most likely cause of the abnormalities. We called the program eDISH (for evaluation of Drug-Induced Serious Hepatotoxicity), and began in 2004 to use it to assess DILI in clinical trial subjects. From 2008, comments made by the presenters at the conferences about their slides and ensuing discussions have been added to the website. All this has raised awareness of the problem, and since 1997, the FDA has not had to withdraw a single drug because of post-marketing hepatotoxicity. Many issues still remain to be resolved; among the most controversial is the best method to estimate likelihood that a given liver injury was actually caused by the drug in question. On November 9, 2012, a workshop was convened to discuss the best practices for the assessment of drug-induced liver injury (DILI) in clinical trials.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical