Methodology to assess clinical liver safety data
- PMID: 25352326
- PMCID: PMC4212156
- DOI: 10.1007/s40264-014-0184-5
Methodology to assess clinical liver safety data
Abstract
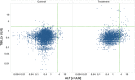
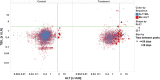
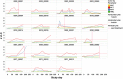
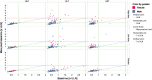
Analysis of liver safety data has to be multivariate by nature and needs to take into account time dependency of observations. Current standard tools for liver safety assessment such as summary tables, individual data listings, and narratives address these requirements to a limited extent only. Using graphics in the context of a systematic workflow including predefined graph templates is a valuable addition to standard instruments, helping to ensure completeness of evaluation, and supporting both hypothesis generation and testing. Employing graphical workflows interactively allows analysis in a team-based setting and facilitates identification of the most suitable graphics for publishing and regulatory reporting. Another important tool is statistical outlier detection, accounting for the fact that for assessment of Drug-Induced Liver Injury, identification and thorough evaluation of extreme values has much more relevance than measures of central tendency in the data. Taken together, systematical graphical data exploration and statistical outlier detection may have the potential to significantly improve assessment and interpretation of clinical liver safety data. A workshop was convened to discuss best practices for the assessment of drug-induced liver injury (DILI) in clinical trials.
Figures

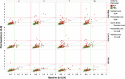

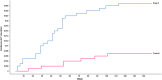



References
-
- US Department of Health and Human Services FDA, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER). Guidance for industry. Drug-induced liver injury: premarketing clinical evaluation. 2009;18.
-
- Krause A, O’Connell M. A picture is worth a thousand tables: graphics in life sciences. New York: Springer; 2012.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

