Alpha-synuclein and tau: teammates in neurodegeneration?
- PMID: 25352339
- PMCID: PMC4230508
- DOI: 10.1186/1750-1326-9-43
Alpha-synuclein and tau: teammates in neurodegeneration?
Abstract
The accumulation of α-synuclein aggregates is the hallmark of Parkinson's disease, and more generally of synucleinopathies. The accumulation of tau aggregates however is classically found in the brains of patients with dementia, and this type of neuropathological feature specifically defines the tauopathies. Nevertheless, in numerous cases α-synuclein positive inclusions are also described in tauopathies and vice versa, suggesting a co-existence or crosstalk of these proteinopathies. Interestingly, α-synuclein and tau share striking common characteristics suggesting that they may work in concord. Tau and α-synuclein are both partially unfolded proteins that can form toxic oligomers and abnormal intracellular aggregates under pathological conditions. Furthermore, mutations in either are responsible for severe dominant familial neurodegeneration. Moreover, tau and α-synuclein appear to promote the fibrillization and solubility of each other in vitro and in vivo. This suggests that interactions between tau and α-synuclein form a deleterious feed-forward loop essential for the development and spreading of neurodegeneration. Here, we review the recent literature with respect to elucidating the possible links between α-synuclein and tau.
Figures


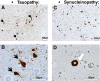
 ), and of αsyn protein, called Lewy bodies and neurites (C and D,
), and of αsyn protein, called Lewy bodies and neurites (C and D,
 ) are often found in neurons of the amygdala in DLB patients.
) are often found in neurons of the amygdala in DLB patients.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

