Invariant natural killer T cells in lupus patients promote IgG and IgG autoantibody production
- PMID: 25352488
- PMCID: PMC4324163
- DOI: 10.1002/eji.201444760
Invariant natural killer T cells in lupus patients promote IgG and IgG autoantibody production
Abstract
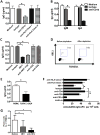
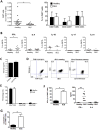
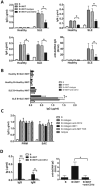
IgG autoantibodies, including antibodies to double-stranded DNA (dsDNA), are pathogenic in systemic lupus erythematosus (SLE), but the mechanisms controlling their production are not understood. To assess the role of invariant natural killer T (iNKT) cells in this process, we studied 44 lupus patients. We took advantage of the propensity of PBMCs from patients with active disease to spontaneously secrete IgG in vitro. Despite the rarity of iNKT cells in lupus blood (0.002-0.05% of CD3-positive T cells), antibody blockade of the conserved iNKT TCR or its ligand, CD1d, or selective depletion of iNKT cells, inhibited spontaneous secretion of total IgG and anti-dsDNA IgG by lupus PBMCs. Addition of anti-iNKT or anti-CD1d antibody to PBMC cultures also reduced the frequency of plasma cells, suggesting that lupus iNKT cells induce B-cell maturation. Like fresh iNKT cells, expanded iNKT-cell lines from lupus patients, but not healthy subjects, induced autologous B cells to secrete antibodies, including IgG anti-dsDNA. This activity was inhibited by anti-CD40L antibody, as well as anti-CD1d antibody, confirming a role for CD40L-CD40 and TCR-CD1d interactions in lupus iNKT-cell-mediated help. These results reveal a critical role for iNKT cells in B-cell maturation and autoantibody production in patients with lupus.
Keywords: Autoantibodies; B cells; IFN-γ; Lupus; iNKT cells.
© 2014 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures

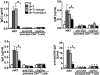

Similar articles
-
Ly108 expression distinguishes subsets of invariant NKT cells that help autoantibody production and secrete IL-21 from those that secrete IL-17 in lupus prone NZB/W mice.J Autoimmun. 2014 May;50:87-98. doi: 10.1016/j.jaut.2014.01.002. Epub 2014 Feb 7. J Autoimmun. 2014. PMID: 24508410 Free PMC article.
-
Invariant NKT cells inhibit autoreactive B cells in a contact- and CD1d-dependent manner.J Immunol. 2011 Feb 1;186(3):1512-20. doi: 10.4049/jimmunol.1002373. Epub 2011 Jan 5. J Immunol. 2011. PMID: 21209282 Free PMC article.
-
Lipid-antigen presentation by CD1d(+) B cells is essential for the maintenance of invariant natural killer T cells.Immunity. 2012 Mar 23;36(3):477-90. doi: 10.1016/j.immuni.2012.02.008. Epub 2012 Mar 8. Immunity. 2012. PMID: 22406267 Free PMC article.
-
Turned on by danger: activation of CD1d-restricted invariant natural killer T cells.Immunology. 2012 Sep;137(1):20-7. doi: 10.1111/j.1365-2567.2012.03612.x. Immunology. 2012. PMID: 22734667 Free PMC article. Review.
-
The role of iNKT cells in the immunopathology of systemic lupus erythematosus.Ann N Y Acad Sci. 2009 Sep;1173:435-41. doi: 10.1111/j.1749-6632.2009.04743.x. Ann N Y Acad Sci. 2009. PMID: 19758183 Review.
Cited by
-
Generation and characterization of CD1d-specific single-domain antibodies with distinct functional features.Immunology. 2016 Sep;149(1):111-21. doi: 10.1111/imm.12635. Immunology. 2016. PMID: 27312006 Free PMC article.
-
Linking CD1-Restricted T Cells With Autoimmunity and Dyslipidemia: Lipid Levels Matter.Front Immunol. 2018 Jul 16;9:1616. doi: 10.3389/fimmu.2018.01616. eCollection 2018. Front Immunol. 2018. PMID: 30061888 Free PMC article. Review.
-
Activation and Regulation of B Cell Responses by Invariant Natural Killer T Cells.Front Immunol. 2018 Jun 18;9:1360. doi: 10.3389/fimmu.2018.01360. eCollection 2018. Front Immunol. 2018. PMID: 29967611 Free PMC article. Review.
-
A Critical Role for Mucosal-Associated Invariant T Cells as Regulators and Therapeutic Targets in Systemic Lupus Erythematosus.Front Immunol. 2019 Nov 29;10:2681. doi: 10.3389/fimmu.2019.02681. eCollection 2019. Front Immunol. 2019. PMID: 31849932 Free PMC article.
-
Regadenoson for the treatment of COVID-19: A five case clinical series and mouse studies.PLoS One. 2023 Aug 11;18(8):e0288920. doi: 10.1371/journal.pone.0288920. eCollection 2023. PLoS One. 2023. PMID: 37566593 Free PMC article.
References
-
- La Cava A, Fang CJ, Singh RP, Ebling F, Hahn BH. Manipulation of immune regulation in systemic lupus erythematosus. Autoimmun Rev. 2005;4:515–519. - PubMed
-
- Ando DG, Sercarz EE, Hahn BH. Mechanisms of T and B cell collaboration in the in vitro production of anti-DNA antibodies in the NZB/NZW F1 murine SLE model. J Immunol. 1987;138:3185–3190. - PubMed
-
- Yuan D, Wilder J, Dang T, Bennett M, Kumar V. Activation of B lymphocytes by NK cells. Int Immunol. 1992;4:1373–1380. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

