Increased dihydroceramide/ceramide ratio mediated by defective expression of degs1 impairs adipocyte differentiation and function
- PMID: 25352638
- PMCID: PMC9757540
- DOI: 10.2337/db14-0359
Increased dihydroceramide/ceramide ratio mediated by defective expression of degs1 impairs adipocyte differentiation and function
Abstract
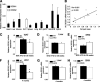
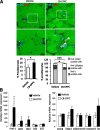
Adipose tissue dysfunction is an important determinant of obesity-associated, lipid-induced metabolic complications. Ceramides are well-known mediators of lipid-induced insulin resistance in peripheral organs such as muscle. DEGS1 is the desaturase catalyzing the last step in the main ceramide biosynthetic pathway. Functional suppression of DEGS1 activity results in substantial changes in ceramide species likely to affect fundamental biological functions such as oxidative stress, cell survival, and proliferation. Here, we show that degs1 expression is specifically decreased in the adipose tissue of obese patients and murine models of genetic and nutritional obesity. Moreover, loss-of-function experiments using pharmacological or genetic ablation of DEGS1 in preadipocytes prevented adipogenesis and decreased lipid accumulation. This was associated with elevated oxidative stress, cellular death, and blockage of the cell cycle. These effects were coupled with increased dihydroceramide content. Finally, we validated in vivo that pharmacological inhibition of DEGS1 impairs adipocyte differentiation. These data identify DEGS1 as a new potential target to restore adipose tissue function and prevent obesity-associated metabolic disturbances.
© 2015 by the American Diabetes Association. Readers may use this article as long as the work is properly cited, the use is educational and not for profit, and the work is not altered.
Figures





References
-
- Montague CT, O’Rahilly S. The perils of portliness: causes and consequences of visceral adiposity. Diabetes 2000;49:883–888 - PubMed
-
- Carobbio S, Rodriguez-Cuenca S, Vidal-Puig A. Origins of metabolic complications in obesity: ectopic fat accumulation. The importance of the qualitative aspect of lipotoxicity. Curr Opin Clin Nutr Metab Care 2011;14:520–526 - PubMed
-
- Chavez JA, Summers SA. A ceramide-centric view of insulin resistance. Cell Metab 2012;15:585–594 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

