The mechanism of Torsin ATPase activation
- PMID: 25352667
- PMCID: PMC4234599
- DOI: 10.1073/pnas.1415271111
The mechanism of Torsin ATPase activation
Abstract
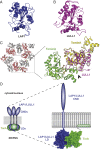
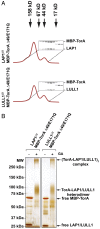
Torsins are membrane-associated ATPases whose activity is dependent on two activating cofactors, lamina-associated polypeptide 1 (LAP1) and luminal domain-like LAP1 (LULL1). The mechanism by which these cofactors regulate Torsin activity has so far remained elusive. In this study, we identify a conserved domain in these activators that is predicted to adopt a fold resembling an AAA+ (ATPase associated with a variety of cellular activities) domain. Within these domains, a strictly conserved Arg residue present in both activating cofactors, but notably missing in Torsins, aligns with a key catalytic Arg found in AAA+ proteins. We demonstrate that cofactors and Torsins associate to form heterooligomeric assemblies with a defined Torsin-activator interface. In this arrangement, the highly conserved Arg residue present in either cofactor comes into close proximity with the nucleotide bound in the neighboring Torsin subunit. Because this invariant Arg is strictly required to stimulate Torsin ATPase activity but is dispensable for Torsin binding, we propose that LAP1 and LULL1 regulate Torsin ATPase activity through an active site complementation mechanism.
Keywords: ATPase; DYT1 dystonia; Torsin; nuclear envelope.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Neuwald AF, Aravind L, Spouge JL, Koonin EV. AAA+: A class of chaperone-like ATPases associated with the assembly, operation, and disassembly of protein complexes. Genome Res. 1999;9(1):27–43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

