Review: neuroestrogen regulation of socio-sexual behavior of males
- PMID: 25352775
- PMCID: PMC4195287
- DOI: 10.3389/fnins.2014.00323
Review: neuroestrogen regulation of socio-sexual behavior of males
Abstract
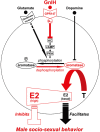
It is thought that estrogen (neuroestrogen) synthesized by the action of aromatase in the brain from testosterone activates male socio-sexual behaviors, such as aggression and sexual behavior in birds. We recently found that gonadotropin-inhibitory hormone (GnIH), a hypothalamic neuropeptide, inhibits socio-sexual behaviors of male quail by directly activating aromatase and increasing neuroestrogen synthesis in the preoptic area (POA). The POA is thought to be the most critical site of aromatization and neuroestrogen action for the regulation of socio-sexual behavior of male birds. We concluded that GnIH inhibits socio-sexual behaviors of male quail by increasing neuroestrogen concentration beyond its optimal concentration in the brain for expression of socio-sexual behavior. On the other hand, it has been reported that dopamine and glutamate, which stimulate male socio-sexual behavior in birds and mammals, inhibit the activity of aromatase in the POA. Multiple studies also report that the activity of aromatase or neuroestrogen is negatively correlated with changes in male socio-sexual behavior in fish, birds, and mammals including humans. Here, we review previous studies that investigated the role of neuroestrogen in the regulation of male socio-sexual behavior and reconsider the hypothesis that neuroestrogen activates male socio-sexual behavior in vertebrates. It is considered that basal concentration of neuroestrogen is required for the maintenance of male socio-sexual behavior but higher concentration of neuroestrogen may inhibit male socio-sexual behavior.
Keywords: aggressive behavior; aromatase; dopamine; glutamate; gonadotropin-inhibitory hormone; neuroestrogen; sexual behavior; socio-sexual behavior.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

