Altered cross-bridge properties in skeletal muscle dystrophies
- PMID: 25352808
- PMCID: PMC4196474
- DOI: 10.3389/fphys.2014.00393
Altered cross-bridge properties in skeletal muscle dystrophies
Abstract
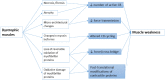
Force and motion generated by skeletal muscle ultimately depends on the cyclical interaction of actin with myosin. This mechanical process is regulated by intracellular Ca(2+) through the thin filament-associated regulatory proteins i.e.; troponins and tropomyosin. Muscular dystrophies are a group of heterogeneous genetic affections characterized by progressive degeneration and weakness of the skeletal muscle as a consequence of loss of muscle tissue which directly reduces the number of potential myosin cross-bridges involved in force production. Mutations in genes responsible for skeletal muscle dystrophies (MDs) have been shown to modify the function of contractile proteins and cross-bridge interactions. Altered gene expression or RNA splicing or post-translational modifications of contractile proteins such as those related to oxidative stress, may affect cross-bridge function by modifying key proteins of the excitation-contraction coupling. Micro-architectural change in myofilament is another mechanism of altered cross-bridge performance. In this review, we provide an overview about changes in cross-bridge performance in skeletal MDs and discuss their ultimate impacts on striated muscle function.
Keywords: cross-bridge kinetics; muscle dystrophy; myopathies; myosin; skeletal muscle.
Figures

References
-
- Baghdiguian S., Martin M., Richard I., Pons F., Astier C., Bourg N., et al. (1999). Calpain 3 deficiency is associated with myonuclear apoptosis and profound perturbation of the IkappaB alpha/NF-kappaB pathway in limb-girdle muscular dystrophy type 2A. Nat. Med. 5, 503–511 10.1038/10579 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

