T cells in vascular inflammatory diseases
- PMID: 25352848
- PMCID: PMC4196542
- DOI: 10.3389/fimmu.2014.00504
T cells in vascular inflammatory diseases
Abstract
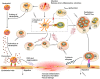
Inflammation of the human vasculature is a manifestation of many different diseases ranging from systemic autoimmune diseases to chronic inflammatory diseases, in which multiple types of immune cells are involved. For both autoimmune diseases and chronic inflammatory diseases several observations support a key role for T lymphocytes in these disease pathologies, but the underlying mechanisms are poorly understood. Previous studies in several autoimmune diseases have demonstrated a significant role for a specific subset of CD4(+) T cells termed effector memory T (TEM) cells. This expanded population of TEM cells may contribute to tissue injury and disease progression. These cells exert multiple pro-inflammatory functions through the release of effector cytokines. Many of these cytokines have been detected in the inflammatory lesions and participate in the vasculitic reaction, contributing to recruitment of macrophages, neutrophils, dendritic cells, natural killer cells, B cells, and T cells. In addition, functional impairment of regulatory T cells paralyzes anti-inflammatory effects in vasculitic disorders. Interestingly, activation of TEM cells is uniquely dependent on the voltage-gated potassium Kv1.3 channel providing an anchor for specific drug targeting. In this review, we focus on the CD4(+) T cells in the context of vascular inflammation and describe the evidence supporting the role of different T cell subsets in vascular inflammation. Selective targeting of pathogenic TEM cells might enable a more tailored therapeutic approach that avoids unwanted adverse side effects of generalized immunosuppression by modulating the effector functions of T cell responses to inhibit the development of vascular inflammation.
Keywords: ANCA-associated vasculitis; Kv1.3 channels; T lymphocytes; atherosclerosis; effector memory T cells; vascular inflammation.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

