A multicenter pilot study examining the role of circulating tumor cells as a blood-based tumor marker in patients with extensive small-cell lung cancer
- PMID: 25353007
- PMCID: PMC4196518
- DOI: 10.3389/fonc.2014.00271
A multicenter pilot study examining the role of circulating tumor cells as a blood-based tumor marker in patients with extensive small-cell lung cancer
Abstract
Background: Small-cell lung cancer (SCLC), a variant of lung cancer marked by early metastases, accounts for 13% of all lung cancers diagnosed in US. Despite high response rates to treatment, it is an aggressive disease with a median survival of 9-11 months for patients with extensive stage (EX-SCLC). Detection of circulating tumor cells (CTCs) is a novel laboratory technique currently in use to determine response to therapy and to predict prognosis in breast, colorectal, and prostate cancer. We initiated a pilot study to analyze the role of CTCs as a biomarker of response and relapse in patients with EX-SCLC.
Methods: We collected blood samples from chemotherapy naïve patients with EX-SCLC prior to initiation of therapy, after completion of systemic therapy, and follow-up every 6-8 weeks and at relapse. The number of CTCs was determined using the cell search system in a central laboratory. The study was conducted in four different sites, and it was reviewed and approved by respective research review committees and IRBs.
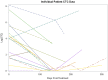
Results: We enrolled 26 patients with EX-SCLC, 1 was excluded due to ineligibility, all were treated with platinum and etoposide. We observed partial response in 16 patients, stable disease in 3 patients, 1 patient with disease progression, and 6 patients were not assessed (5 deceased, 1 not available). The overall median number of CTCs in 24 patients measured at baseline and post-tx was 75 (range 0-3430) and 2 (range 0-526), respectively. A significant reduction in CTCs from baseline to post-treatment was identified for 15 subjects; the median reduction was 97.4% (range -100 to +100%, p < 0.001). Higher baseline CTCs and percentage change in post-treatment CTCs were associated with decreased survival.
Conclusion: We demonstrated that it is feasible to detect CTCs in EX-SCLC. If validated in other prospective studies, CTCs could be a useful biomarker in the management of EX-SCLC by predicting patients' clinical responses to therapy.
Keywords: biomarkers; circulating tumor cells; extensive stage; prognosis; small-cell lung carcinoma.
Figures



References
-
- Govindan R, Page N, Morgensztern D, Read W, Tierney R, Vlahiotis A, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol (2006) 24(28):4539–44 - PubMed
-
- Sundstrøm S, Bremnes RM, Kaasa S, Aasebø U, Hatlevoll R, Dahle R, et al. Cisplatin and etoposide regimen is superior to cyclyfophosphamide, epirubicin, and vincrisitine regimen in small-cell lung cancer: results from a randomized phase III trial with 5 years’ follow up. J Clin Oncol (2002) 20(24):4665–7210.1200/JCO.2002.12.111 - DOI - PubMed
-
- Cohen SJ, Punt CJ, Iannotti N, Saidman BH, Sabbath KD, Gabrail NY, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol (2008) 26(19):3213–2110.1200/JCO.2007.15.8923 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

