Nasal screening for MRSA: different swabs--different results!
- PMID: 25353631
- PMCID: PMC4213029
- DOI: 10.1371/journal.pone.0111627
Nasal screening for MRSA: different swabs--different results!
Abstract
Objectives: Swab-based nasal screening is commonly used to identify asymptomatic carriage of Staphylococcus aureus in patients. Bacterial detection depends on the uptake and release capacities of the swabs and on the swabbing technique itself. This study investigates the performance of different swab-types in nasal MRSA-screening by utilizing a unique artificial nose model to provide realistic and standardized screening conditions.
Methods: An anatomically correct artificial nose model was inoculated with a numerically defined mixture of MRSA and Staphylococcus epidermidis bacteria at quantities of 4×10(2) and 8×10(2) colony forming units (CFU), respectively. Five swab-types were tested following a strict protocol. Bacterial recovery was measured for direct plating and after elution into Amies medium by standard viable count techniques.
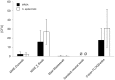
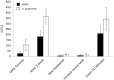
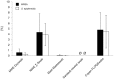
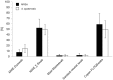
Results: Mean recovered bacteria quantities varied between 209 and 0 CFU for MRSA, and 365 and 0 CFU for S. epidermidis, resulting swab-type-dependent MRSA-screening-sensitivities ranged between 0 and 100%. Swabs with nylon flocked tips or cellular foam tips performed significantly better compared to conventional rayon swabs referring to the recovered bacterial yield (p<0.001). Best results were obtained by using a flocked swab in combination with Amies preservation medium. Within the range of the utilized bacterial concentrations, recovery ratios for the particular swab-types were independent of the bacterial species.
Conclusions: This study combines a realistic model of a human nose with standardized laboratory conditions to analyze swab-performance in MRSA-screening situations. Therefore, influences by inter-individual anatomical differences as well as diverse colonization densities in patients could be excluded. Recovery rates vary significantly between different swab-types. The choice of the swab has a great impact on the laboratory result. In fact, the swab-type contributes significantly to true positive or false negative detection of nasal MRSA carriage. These findings should be considered when screening a patient.
Conflict of interest statement
Figures
References
-
- Wertheim HF, Melles DC, Vos MC, van Leeuwen W, van Belkum A, et al. (2005) The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis 5: 751–762. - PubMed
-
- Kluytmans JA, Wertheim HF (2005) Nasal carriage of Staphylococcus aureus and prevention of nosocomial infections. Infection 33: 3–8. - PubMed
-
- Peacock SJ, de Silva I, Lowy FD (2001) What determines nasal carriage of Staphylococcus aureus? Trends Microbiol 9: 605–610. - PubMed
-
- Davis KA, Stewart JJ, Crouch HK, Florez CE, Hospenthal DR (2004) Methicillin-resistant Staphylococcus aureus (MRSA) nares colonization at hospital admission and its effect on subsequent MRSA infection. Clin Infect Dis 39: 776–782. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

