Clinical illness and outcomes in patients with Ebola in Sierra Leone
- PMID: 25353969
- PMCID: PMC4318555
- DOI: 10.1056/NEJMoa1411680
Clinical illness and outcomes in patients with Ebola in Sierra Leone
Abstract
Background: Limited clinical and laboratory data are available on patients with Ebola virus disease (EVD). The Kenema Government Hospital in Sierra Leone, which had an existing infrastructure for research regarding viral hemorrhagic fever, has received and cared for patients with EVD since the beginning of the outbreak in Sierra Leone in May 2014.
Methods: We reviewed available epidemiologic, clinical, and laboratory records of patients in whom EVD was diagnosed between May 25 and June 18, 2014. We used quantitative reverse-transcriptase-polymerase-chain-reaction assays to assess the load of Ebola virus (EBOV, Zaire species) in a subgroup of patients.
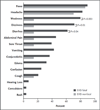
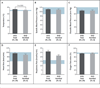
Results: Of 106 patients in whom EVD was diagnosed, 87 had a known outcome, and 44 had detailed clinical information available. The incubation period was estimated to be 6 to 12 days, and the case fatality rate was 74%. Common findings at presentation included fever (in 89% of the patients), headache (in 80%), weakness (in 66%), dizziness (in 60%), diarrhea (in 51%), abdominal pain (in 40%), and vomiting (in 34%). Clinical and laboratory factors at presentation that were associated with a fatal outcome included fever, weakness, dizziness, diarrhea, and elevated levels of blood urea nitrogen, aspartate aminotransferase, and creatinine. Exploratory analyses indicated that patients under the age of 21 years had a lower case fatality rate than those over the age of 45 years (57% vs. 94%, P=0.03), and patients presenting with fewer than 100,000 EBOV copies per milliliter had a lower case fatality rate than those with 10 million EBOV copies per milliliter or more (33% vs. 94%, P=0.003). Bleeding occurred in only 1 patient.
Conclusions: The incubation period and case fatality rate among patients with EVD in Sierra Leone are similar to those observed elsewhere in the 2014 outbreak and in previous outbreaks. Although bleeding was an infrequent finding, diarrhea and other gastrointestinal manifestations were common. (Funded by the National Institutes of Health and others.).
Figures


References
-
- World Health Organization. Ebola response roadmap situation report. 2014 Oct 25; ( http://www.who.int/csr/disease/ebola/situation-reports/en).
-
- McCarthy M. Ebola is diagnosed in traveler to US. BMJ. 2014;349:g5980. - PubMed
-
- Baize S, Pannetier D, Oestereich L, et al. Emergence of Zaire Ebola virus disease in Guinea. N Engl J Med. 2014;371:1418–1425. - PubMed
Publication types
MeSH terms
Grants and funding
- HHSN272201000022C/AI/NIAID NIH HHS/United States
- 1U01HG007480-01/HG/NHGRI NIH HHS/United States
- 1R01AI104621/AI/NIAID NIH HHS/United States
- T32 GM080177/GM/NIGMS NIH HHS/United States
- P20 RR021970/RR/NCRR NIH HHS/United States
- R44 AI088843/AI/NIAID NIH HHS/United States
- P20 GM103501/GM/NIGMS NIH HHS/United States
- R01 AI104621/AI/NIAID NIH HHS/United States
- DP2 OD006514/OD/NIH HHS/United States
- R01 AI099699/AI/NIAID NIH HHS/United States
- HHSN272200900049C/AI/NIAID NIH HHS/United States
- 2R44AI088843/AI/NIAID NIH HHS/United States
- U19 AI115589/AI/NIAID NIH HHS/United States
- P20GM103501/GM/NIGMS NIH HHS/United States
- 1DP2OD006514-01/OD/NIH HHS/United States
- 1U19AI109762/AI/NIAID NIH HHS/United States
- U01 HG007480/HG/NHGRI NIH HHS/United States
- 100890/WT_/Wellcome Trust/United Kingdom
- U19 AI109762/AI/NIAID NIH HHS/United States
- GM080177/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
