Primary osteoblast-like cells from patients with end-stage kidney disease reflect gene expression, proliferation, and mineralization characteristics ex vivo
- PMID: 25354236
- PMCID: PMC4344911
- DOI: 10.1038/ki.2014.347
Primary osteoblast-like cells from patients with end-stage kidney disease reflect gene expression, proliferation, and mineralization characteristics ex vivo
Abstract
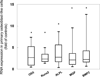
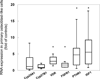
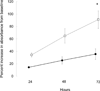
Osteocytes regulate bone turnover and mineralization in chronic kidney disease. As osteocytes are derived from osteoblasts, alterations in osteoblast function may regulate osteoblast maturation, osteocytic transition, bone turnover, and skeletal mineralization. Thus, primary osteoblast-like cells were cultured from bone chips obtained from 24 pediatric ESKD patients. RNA expression in cultured cells was compared with RNA expression in cells from healthy individuals, to RNA expression in the bone core itself, and to parameters of bone histomorphometry. Proliferation and mineralization rates of patient cells were compared with rates in healthy control cells. Associations were observed between bone osteoid accumulation, as assessed by bone histomorphometry, and bone core RNA expression of osterix, matrix gla protein, parathyroid hormone receptor 1, and RANKL. Gene expression of osteoblast markers was increased in cells from ESKD patients and signaling genes including Cyp24A1, Cyp27B1, VDR, and NHERF1 correlated between cells and bone cores. Cells from patients with high turnover renal osteodystrophy proliferated more rapidly and mineralized more slowly than did cells from healthy controls. Thus, primary osteoblasts obtained from patients with ESKD retain changes in gene expression ex vivo that are also observed in bone core specimens. Evaluation of these cells in vitro may provide further insights into the abnormal bone biology that persists, despite current therapies, in patients with ESKD.
Figures




Comment in
-
Bone cells, sclerostin, and FGF23: what's bred in the bone will come out in the flesh.Kidney Int. 2015 Mar;87(3):499-501. doi: 10.1038/ki.2014.360. Kidney Int. 2015. PMID: 25723633
-
Does bone structure accurately reflect serum FGF23 levels in patients with chronic kidney disease?Kidney Int. 2015 Sep;88(3):640. doi: 10.1038/ki.2015.206. Kidney Int. 2015. PMID: 26323079 No abstract available.
-
The Authors Reply.Kidney Int. 2015 Sep;88(3):640-1. doi: 10.1038/ki.2015.219. Kidney Int. 2015. PMID: 26323080 No abstract available.
References
-
- Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, Fujita T, Nakahara K, Fukumoto S, Yamashita T. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J.Bone Miner.Res. 2004;19:429–435. - PubMed
-
- Sabbagh Y, Graciolli FG, O'Brien S, Tang W, Dos Reis LM, Ryan S, Phillips L, Boulanger J, Song W, Bracken C, Liu S, Ledbetter S, Dechow P, Canziani ME, Carvalho AB, Jorgetti V, Moyses RM, Schiavi SC. Repression of osteocyte Wnt/beta-catenin signaling is an early event in the progression of renal osteodystrophy. J Bone Miner Res. 2012;27:1757–1772. - PubMed
-
- Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, Gutierrez OM, Aguillon-Prada R, Lincoln J, Hare JM, Mundel P, Morales A, Scialla J, Fischer M, Soliman EZ, Chen J, Go AS, Rosas SE, Nessel L, Townsend RR, Feldman HI, St John Sutton M, Ojo A, Gadegbeku C, Di Marco GS, Reuter S, Kentrup D, Tiemann K, Brand M, Hill JA, Moe OW, Kuro OM, Kusek JW, Keane MG, Wolf M. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121:4393–4408. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

