Cellular and molecular immune profiles in renal transplant recipients after conversion from tacrolimus to sirolimus
- PMID: 25354238
- PMCID: PMC4382402
- DOI: 10.1038/ki.2014.350
Cellular and molecular immune profiles in renal transplant recipients after conversion from tacrolimus to sirolimus
Abstract
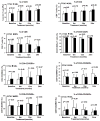
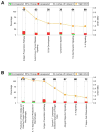
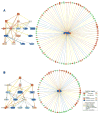
Tacrolimus and sirolimus are commonly used maintenance immunosuppressants in kidney transplantation. As their effects on immune cells and allograft molecular profiles have not been elucidated, we characterized the effects of tacrolimus to sirolimus conversion on the frequency and function of T cells, and on graft molecular profiles. Samples from renal transplant patients in a randomized trial of 18 patients with late sirolimus conversion and 12 on tacrolimus maintenance were utilized. Peripheral blood was collected at 0, 6, 12, and 24 months post randomization, with T-cell subpopulations analyzed by flow cytometry and T-cell alloreactivity tested by IFN-γ ELISPOT. Graft biopsy samples obtained 24 months post randomization were used for gene expression analysis. Sirolimus conversion led to an increase in CD4(+)25(+++)Foxp3(+) regulatory T cells. While tacrolimus-maintained patients showed a decrease in indirect alloreactivity over time post transplant, sirolimus conversion increased indirect alloreactive T-cell frequencies compared with tacrolimus-maintained patients. No histological differences were found in graft biopsies, but molecular profiles showed activation of the antigen presentation, IL-12 signaling, oxidative stress, macrophage-derived production pathways, and increased inflammatory and immune response in sirolimus-converted patients. Thus, chronic immune alterations are induced after sirolimus conversion. Despite the molecular profile being favorable to calcineurin inhibitor-based regimen, there was no impact in renal function over 30 months of follow-up.
Conflict of interest statement
The authors do not have any conflicts of interest to disclose.
Figures



References
-
- Meier-Kriesche HU, Schold JD, Srinivas TR, et al. Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2004;4:378–383. - PubMed
-
- Grinyo JM, Bestard O, Torras J, et al. Optimal immunosuppression to prevent chronic allograft dysfunction. Kidney Int Suppl. 2010:S66–70. - PubMed
-
- Mannon RB. Therapeutic targets in the treatment of allograft fibrosis. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2006;6:867–875. - PubMed
-
- Racusen LC, Solez K, Colvin RB, et al. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999;55:713–723. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

