A reassessment of soluble urokinase-type plasminogen activator receptor in glomerular disease
- PMID: 25354239
- PMCID: PMC4344842
- DOI: 10.1038/ki.2014.346
A reassessment of soluble urokinase-type plasminogen activator receptor in glomerular disease
Abstract
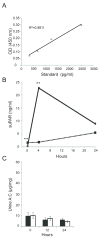
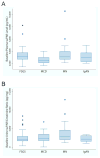
It has been suggested that soluble urokinase receptor (suPAR) is a causative circulating factor for and a biomarker of focal and segmental glomerulosclerosis (FSGS). Here we undertook validation of these assumptions in both mouse and human models. Injection of recombinant suPAR in wild-type mice did not induce proteinuria within 24 h. Moreover, a disease phenotype was not seen in an inducible transgenic mouse model that maintained elevated suPAR concentrations for 6 weeks. Plasma and urine suPAR concentrations were evaluated as clinical biomarkers in 241 patients with glomerular disease from the prospective, longitudinal multicenter observational NEPTUNE cohort. The serum suPAR concentration at baseline inversely correlated with estimated glomerular filtration rate (eGFR) and the urine suPAR/creatinine ratio positively correlated with the urine protein/creatinine ratio. After adjusting for eGFR and urine protein, neither the serum nor urine suPAR level was an independent predictor of FSGS histopathology. A multivariable mixed-effects model of longitudinal data evaluated the association between the change in serum suPAR concentration from baseline with eGFR. After adjusting for baseline suPAR concentration, age, gender, proteinuria, and time, the change in suPAR from baseline was associated with eGFR, but this association was not different for patients with FSGS as compared with other diagnoses. Thus these results do not support a pathological role for suPAR in FSGS.
Conflict of interest statement
The authors have no financial relationship with companies that might have interest in the information contained in this manuscript.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

