Structural basis of IL-23 antagonism by an Alphabody protein scaffold
- PMID: 25354530
- PMCID: PMC4220489
- DOI: 10.1038/ncomms6237
Structural basis of IL-23 antagonism by an Alphabody protein scaffold
Abstract
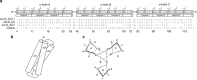
Protein scaffolds can provide a promising alternative to antibodies for various biomedical and biotechnological applications, including therapeutics. Here we describe the design and development of the Alphabody, a protein scaffold featuring a single-chain antiparallel triple-helix coiled-coil fold. We report affinity-matured Alphabodies with favourable physicochemical properties that can specifically neutralize human interleukin (IL)-23, a pivotal therapeutic target in autoimmune inflammatory diseases such as psoriasis and multiple sclerosis. The crystal structure of human IL-23 in complex with an affinity-matured Alphabody reveals how the variable interhelical groove of the scaffold uniquely targets a large epitope on the p19 subunit of IL-23 to harness fully the hydrophobic and hydrogen-bonding potential of tryptophan and tyrosine residues contributed by p19 and the Alphabody, respectively. Thus, Alphabodies are suitable for targeting protein-protein interfaces of therapeutic importance and can be tailored to interrogate desired design and binding-mode principles via efficient selection and affinity-maturation strategies.
Conflict of interest statement
J.D., E.L., K.V., S.L., T.H., S.D., K.S., P.H. and I.L. declare financial interests as employees of COMPLIX N.V. (Ghent, Belgium), a biopharmaceutical company dedicated to the development of the Alphabody scaffold. The remaining authors declare no competing financial interests.
Figures
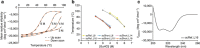
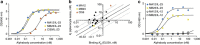



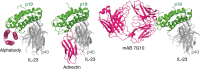
References
-
- Chan A. C. & Carter P. J. Therapeutic antibodies for autoimmunity and inflammation. Nat. Rev. Immunol. 10, 301–316 (2010). - PubMed
-
- Page D. B., Postow M. A., Callahan M. K., Allison J. P. & Wolchok J. D. Immune modulation in cancer with antibodies. Annu. Rev. Med. 65, 185–202 (2014). - PubMed
-
- Wells J. A. & McClendon C. L. Reaching for high-hanging fruit in drug discovery at protein-protein interfaces. Nature 450, 1001–1009 (2007). - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

