Effects of BMP-12-releasing sutures on Achilles tendon healing
- PMID: 25354567
- PMCID: PMC4356234
- DOI: 10.1089/ten.TEA.2014.0001
Effects of BMP-12-releasing sutures on Achilles tendon healing
Abstract
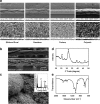
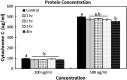
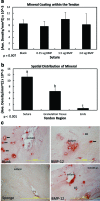
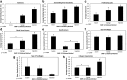
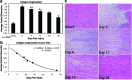
Tendon healing is a complex coordinated event orchestrated by numerous biologically active proteins. Unfortunately, tendons have limited regenerative potential and as a result, repair may be protracted months to years. Current treatment strategies do not offer localized delivery of biologically active proteins, which may result in reduced therapeutic efficacy. Surgical sutures coated with nanostructured minerals may provide a potentially universal tool to efficiently incorporate and deliver biologically active proteins directly to the wound. Additionally, previous reports indicated that treatment with bone morphogenetic protein-12 (BMP-12) improved tendon healing. Based on this information, we hypothesized that mineral-coated surgical sutures may be an effective platform for localized BMP-12 delivery to an injured tendon. The objective of this study was, therefore, to elucidate the healing effects of mineral-coated sutures releasing BMP-12 using a rat Achilles healing model. The effects of BMP-12-releasing sutures were also compared with standard BMP-12 delivery methods, including delivery of BMP-12 through collagen sponge or direct injection. Rat Achilles tendons were unilaterally transected and repaired using BMP-12-releasing suture (0, 0.15, 1.5, or 3.0 μg), collagen sponge (0 or 1.5 μg BMP-12), or direct injection (0 or 1.5 μg). By 14 days postinjury, repair with BMP-12-releasing sutures reduced the appearance of adhesions to the tendon and decreased total cell numbers. BMP-12 released from sutures and collagen sponge also tended to improve collagen organization when compared with BMP-12 delivered through injection. Based on these results, the release of a protein from sutures was able to elicit a biological response. Furthermore, BMP-12-releasing sutures modulated tendon healing, and the delivery method dictated the response of the healing tissue to BMP-12.
Figures
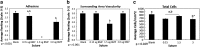

References
-
- Jeon O., Song S.J., Yang H.S., et al. . Long-term delivery enhances in vivo osteogenic efficacy of bone morphogenetic protein-2 compared to short-term delivery. Biochem Biophys Res Commun 369,774, 2008 - PubMed
-
- Zhu G., Mallery S.R., and Schwendeman S.P.Stabilization of proteins encapsulated in injectable poly (lactide-co-glycolide). Nat Biotechnol 18,52, 2000 - PubMed
-
- Zurita R., Puiggali J., and Rodriguez-Galan A.Loading and release of ibuprofen in multi- and monofilament surgical sutures. Macromol Biosci 6,767, 2006 - PubMed
-
- Lee J.S., Lu Y., Baer G.S., et al. . Controllable protein delivery from coated surgical sutures. J Mater Chem 20,8894, 2010
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

