Isolation of esophageal stem cells with potential for therapy
- PMID: 25354803
- PMCID: PMC4229645
- DOI: 10.1007/s00383-014-3615-6
Isolation of esophageal stem cells with potential for therapy
Abstract
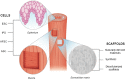
Purpose: Long-gap esophageal atresia represents a significant challenge for pediatric surgeons and current surgical approaches are associated with significant morbidity. A tissue-engineered esophagus, comprising cells seeded onto a scaffold, represents a therapeutic alternative. In this study, we aimed to determine the optimal techniques for isolation and culture of mouse esophageal epithelial cells and to isolate CD34-positive esophageal epithelial stem cells from cadaveric mouse specimens.
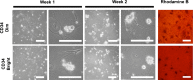
Methods: Primary epithelial cells were isolated from mouse esophagi by enzymatic dissociation from the mucosal layer (Dispase, Trypsin/EDTA) using three different protocols. In protocol A, isolated mucosa was minced and incubated with trypsin once. In protocol B, intact mucosal sheets underwent two trypsin incubations yielding a single-cell suspension. In protocol C, intact mucosa explants were plated epithelial side down. Epithelial cells were cultured on collagen-coated wells.
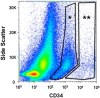
Results: Initial findings showed that Protocol B gave the best results in terms of yield, viability, and least contamination with different cell types and microbes. Esophageal epithelial cells isolated using Protocol B were stained for CD34 and sorted using fluorescence-activated cell sorting (FACS). Of the total cells sorted, 8.3% (2-11.3) [%median (range)] were CD34 positive.
Conclusions: Our results demonstrate that mouse esophageal epithelial cells can be successfully isolated from fresh mouse esophagi using two consecutive trypsin incubations of intact mucosal sheets. Furthermore, the cells obtained using this method were successfully stained for CD34, a putative esophageal epithelial stem cell marker. Further research into the factors necessary for the successful proliferation of CD34 positive stem cell lines is needed to progress toward clinical application.
Figures



Similar articles
-
Esophagus tissue engineering: in vitro generation of esophageal epithelial cell sheets and viability on scaffold.J Pediatr Surg. 2009 May;44(5):896-901. doi: 10.1016/j.jpedsurg.2009.01.019. J Pediatr Surg. 2009. PMID: 19433165
-
Culture of ovine esophageal epithelial cells and in vitro esophagus tissue engineering.Tissue Eng Part C Methods. 2010 Feb;16(1):109-14. doi: 10.1089/ten.TEC.2009.0145. Tissue Eng Part C Methods. 2010. PMID: 19374530
-
Polyurethane scaffolds seeded with autologous cells can regenerate long esophageal gaps: An esophageal atresia treatment model.J Pediatr Surg. 2019 Sep;54(9):1744-1754. doi: 10.1016/j.jpedsurg.2018.09.024. Epub 2018 Oct 22. J Pediatr Surg. 2019. PMID: 30429066
-
Esophagus tissue engineering: designing and crafting the components for the "hybrid construct" approach.Eur J Pediatr Surg. 2014 Jun;24(3):246-62. doi: 10.1055/s-0034-1382261. Epub 2014 Jun 11. Eur J Pediatr Surg. 2014. PMID: 24918402 Review.
-
Decellularized material as scaffolds for tissue engineering studies in long gap esophageal atresia.Expert Opin Biol Ther. 2017 May;17(5):573-584. doi: 10.1080/14712598.2017.1308482. Epub 2017 Mar 27. Expert Opin Biol Ther. 2017. PMID: 28303723 Review.
Cited by
-
Methylsulfonylmethane inhibits cortisol-induced stress through p53-mediated SDHA/HPRT1 expression in racehorse skeletal muscle cells: A primary step against exercise stress.Exp Ther Med. 2020 Jan;19(1):214-222. doi: 10.3892/etm.2019.8196. Epub 2019 Nov 13. Exp Ther Med. 2020. PMID: 31853292 Free PMC article.
-
Cadaveric Stem Cells: Their Research Potential and Limitations.Front Genet. 2021 Dec 22;12:798161. doi: 10.3389/fgene.2021.798161. eCollection 2021. Front Genet. 2021. PMID: 35003228 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

