The fused anthranilate synthase from Streptomyces venezuelae functions as a monomer
- PMID: 25355158
- PMCID: PMC4303589
- DOI: 10.1007/s11010-014-2256-3
The fused anthranilate synthase from Streptomyces venezuelae functions as a monomer
Abstract
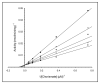
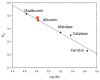
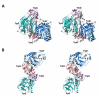
Recently, we showed that the fused chorismate-utilizing enzyme from the antibiotic-producing soil bacterium Streptomyces venezuelae is an anthranilate synthase (designated SvAS), not a 2-amino-2-deoxyisochorismate (ADIC) synthase, as was predicted based on its amino acid sequence similarity to the phenazine biosynthetic enzyme PhzE (an ADIC synthase). Here, we report the characterization of SvAS using steady-state kinetics, gel filtration chromatography, and laser light scattering. The recombinant His-tagged enzyme has Michaelis constants Km with respect to substrates chorismate and glutamine of 8.2 ± 0.2 μM and 0.84 ± 0.05 mM, respectively, and a catalytic rate constant k cat of 0.57 ± 0.02 s(-1) at 30 °C. Unlike most other anthranilate synthases, SvAS does not utilize ammonia as a substrate. The enzyme is competitively but non-cooperatively inhibited by tryptophan (K i = 11.1 ± 0.1 μM) and is active as a monomer. The finding that SvAS is a monomer jibes with the variety of association modes that have been observed for anthranilate synthases from different microorganisms, and it identifies the enzyme's minimal functional unit as a single TrpE-TrpG pair.
Figures


Similar articles
-
The fused TrpEG from Streptomyces venezuelae is an anthranilate synthase, not a 2-amino-2-deoxyisochorismate [corrected] (ADIC) synthase.Ethn Dis. 2008 Spring;18(2 Suppl 2):S2-9-13. Ethn Dis. 2008. PMID: 18646313 Free PMC article.
-
Regulation of an anthranilate synthase gene in Streptomyces venezuelae by a trp attenuator.Microbiology (Reading). 1998 Jul;144 ( Pt 7):1971-1980. doi: 10.1099/00221287-144-7-1971. Microbiology (Reading). 1998. PMID: 9695930
-
Anthranilate synthase without an LLES motif from a hyperthermophilic archaeon is inhibited by tryptophan.Biochem Biophys Res Commun. 2001 Mar 9;281(4):858-65. doi: 10.1006/bbrc.2001.4428. Biochem Biophys Res Commun. 2001. PMID: 11237738
-
Thermodynamics of reactions catalyzed by anthranilate synthase.Biophys Chem. 2000 Feb 14;84(1):45-64. doi: 10.1016/s0301-4622(99)00145-3. Biophys Chem. 2000. PMID: 10723544
-
The structures of anthranilate synthase of Serratia marcescens crystallized in the presence of (i) its substrates, chorismate and glutamine, and a product, glutamate, and (ii) its end-product inhibitor, L-tryptophan.Proc Natl Acad Sci U S A. 2001 May 22;98(11):6021-6. doi: 10.1073/pnas.111150298. Proc Natl Acad Sci U S A. 2001. PMID: 11371633 Free PMC article.
Cited by
-
TrpM, a Small Protein Modulating Tryptophan Biosynthesis and Morpho-Physiological Differentiation in Streptomyces coelicolor A3(2).PLoS One. 2016 Sep 26;11(9):e0163422. doi: 10.1371/journal.pone.0163422. eCollection 2016. PLoS One. 2016. PMID: 27669158 Free PMC article.
-
A Three-Ring Circus: Metabolism of the Three Proteogenic Aromatic Amino Acids and Their Role in the Health of Plants and Animals.Front Mol Biosci. 2018 Apr 6;5:29. doi: 10.3389/fmolb.2018.00029. eCollection 2018. Front Mol Biosci. 2018. PMID: 29682508 Free PMC article. Review.
-
Unraveling the Structure and Mechanism of the MST(ery) Enzymes.Trends Biochem Sci. 2018 May;43(5):342-357. doi: 10.1016/j.tibs.2018.02.011. Epub 2018 Mar 21. Trends Biochem Sci. 2018. PMID: 29573882 Free PMC article. Review.
-
Novel pathway of 3-hydroxyanthranilic acid formation in limazepine biosynthesis reveals evolutionary relation between phenazines and pyrrolobenzodiazepines.Sci Rep. 2018 May 17;8(1):7810. doi: 10.1038/s41598-018-26179-w. Sci Rep. 2018. PMID: 29773836 Free PMC article.
References
-
- Kane JF, Jensen RA. The molecular aggregation of anthranilate synthase in Bacillus subtilis. Biochem Biophys Res Commun. 1970;41:328–333. - PubMed
-
- Sawula RV, Crawford IP. Anthranilate synthetase of Acinetobacter calcoaceticus: separation and partial characterization of subunits. J Biol Chem. 1973;248:3573–3581. - PubMed
-
- Queener SW, Queener SF, Meeks JR, Gunsalus IC. Anthranilate synthase from Pseudomonas putida: purification and properties of a two-component enzyme. J Biol Chem. 1973;248:151–161. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

