The fused anthranilate synthase from Streptomyces venezuelae functions as a monomer
- PMID: 25355158
- PMCID: PMC4303589
- DOI: 10.1007/s11010-014-2256-3
The fused anthranilate synthase from Streptomyces venezuelae functions as a monomer
Abstract
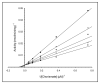
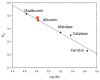
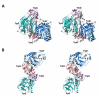
Recently, we showed that the fused chorismate-utilizing enzyme from the antibiotic-producing soil bacterium Streptomyces venezuelae is an anthranilate synthase (designated SvAS), not a 2-amino-2-deoxyisochorismate (ADIC) synthase, as was predicted based on its amino acid sequence similarity to the phenazine biosynthetic enzyme PhzE (an ADIC synthase). Here, we report the characterization of SvAS using steady-state kinetics, gel filtration chromatography, and laser light scattering. The recombinant His-tagged enzyme has Michaelis constants Km with respect to substrates chorismate and glutamine of 8.2 ± 0.2 μM and 0.84 ± 0.05 mM, respectively, and a catalytic rate constant k cat of 0.57 ± 0.02 s(-1) at 30 °C. Unlike most other anthranilate synthases, SvAS does not utilize ammonia as a substrate. The enzyme is competitively but non-cooperatively inhibited by tryptophan (K i = 11.1 ± 0.1 μM) and is active as a monomer. The finding that SvAS is a monomer jibes with the variety of association modes that have been observed for anthranilate synthases from different microorganisms, and it identifies the enzyme's minimal functional unit as a single TrpE-TrpG pair.
Figures


References
-
- Kane JF, Jensen RA. The molecular aggregation of anthranilate synthase in Bacillus subtilis. Biochem Biophys Res Commun. 1970;41:328–333. - PubMed
-
- Sawula RV, Crawford IP. Anthranilate synthetase of Acinetobacter calcoaceticus: separation and partial characterization of subunits. J Biol Chem. 1973;248:3573–3581. - PubMed
-
- Queener SW, Queener SF, Meeks JR, Gunsalus IC. Anthranilate synthase from Pseudomonas putida: purification and properties of a two-component enzyme. J Biol Chem. 1973;248:151–161. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

