Can antioxidants protect against disuse muscle atrophy?
- PMID: 25355189
- PMCID: PMC4213375
- DOI: 10.1007/s40279-014-0255-x
Can antioxidants protect against disuse muscle atrophy?
Abstract
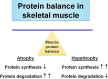
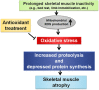
Long periods of skeletal muscle inactivity (e.g. prolonged bed rest or limb immobilization) results in a loss of muscle protein and fibre atrophy. This disuse-induced muscle atrophy is due to both a decrease in protein synthesis and increased protein breakdown. Although numerous factors contribute to the regulation of the rates of protein breakdown and synthesis in skeletal muscle, it has been established that prolonged muscle inactivity results in increased radical production in the inactive muscle fibres. Further, this increase in radical production plays an important role in the regulation of redox-sensitive signalling pathways that regulate both protein synthesis and proteolysis in skeletal muscle. Indeed, it was suggested over 20 years ago that antioxidant supplementation has the potential to protect skeletal muscles against inactivity-induced fibre atrophy. Since this original proposal, experimental evidence has implied that a few compounds with antioxidant properties are capable of delaying inactivity-induced muscle atrophy. The objective of this review is to discuss the role that radicals play in the regulation of inactivity-induced skeletal muscle atrophy and to provide an analysis of the recent literature indicating that specific antioxidants have the potential to defer disuse muscle atrophy.
Figures
Similar articles
-
Redox Control of Proteolysis During Inactivity-Induced Skeletal Muscle Atrophy.Antioxid Redox Signal. 2020 Sep 10;33(8):559-569. doi: 10.1089/ars.2019.8000. Epub 2020 Feb 24. Antioxid Redox Signal. 2020. PMID: 31941357 Free PMC article. Review.
-
Immobilization-induced activation of key proteolytic systems in skeletal muscles is prevented by a mitochondria-targeted antioxidant.J Appl Physiol (1985). 2013 Aug 15;115(4):529-38. doi: 10.1152/japplphysiol.00471.2013. Epub 2013 Jun 13. J Appl Physiol (1985). 2013. PMID: 23766499
-
Oxidative stress and disuse muscle atrophy: cause or consequence?Curr Opin Clin Nutr Metab Care. 2012 May;15(3):240-5. doi: 10.1097/MCO.0b013e328352b4c2. Curr Opin Clin Nutr Metab Care. 2012. PMID: 22466926 Free PMC article. Review.
-
Mitochondrial-targeted antioxidants protect skeletal muscle against immobilization-induced muscle atrophy.J Appl Physiol (1985). 2011 Nov;111(5):1459-66. doi: 10.1152/japplphysiol.00591.2011. Epub 2011 Aug 4. J Appl Physiol (1985). 2011. PMID: 21817113 Free PMC article.
-
Redox control of skeletal muscle atrophy.Free Radic Biol Med. 2016 Sep;98:208-217. doi: 10.1016/j.freeradbiomed.2016.02.021. Epub 2016 Feb 18. Free Radic Biol Med. 2016. PMID: 26912035 Free PMC article. Review.
Cited by
-
Effect of Quercetin on Dexamethasone-Induced C2C12 Skeletal Muscle Cell Injury.Molecules. 2020 Jul 17;25(14):3267. doi: 10.3390/molecules25143267. Molecules. 2020. PMID: 32709024 Free PMC article.
-
Ubiquitin-proteasome pathway in skeletal muscle atrophy.Front Physiol. 2023 Nov 17;14:1289537. doi: 10.3389/fphys.2023.1289537. eCollection 2023. Front Physiol. 2023. PMID: 38046952 Free PMC article. Review.
-
Mechanistic Role of Reactive Oxygen Species and Therapeutic Potential of Antioxidants in Denervation- or Fasting-Induced Skeletal Muscle Atrophy.Front Physiol. 2018 Mar 14;9:215. doi: 10.3389/fphys.2018.00215. eCollection 2018. Front Physiol. 2018. PMID: 29593571 Free PMC article.
-
Eldecalcitol prevents muscle loss and osteoporosis in disuse muscle atrophy via NF-κB signaling in mice.Skelet Muscle. 2023 Dec 19;13(1):22. doi: 10.1186/s13395-023-00332-0. Skelet Muscle. 2023. PMID: 38115079 Free PMC article.
-
Reactive oxygen species-scavenging nanomaterials for the prevention and treatment of age-related diseases.J Nanobiotechnology. 2024 May 15;22(1):252. doi: 10.1186/s12951-024-02501-9. J Nanobiotechnology. 2024. PMID: 38750509 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

