A randomized, double-blind, placebo-controlled study of breath powered nasal delivery of sumatriptan powder (AVP-825) in the treatment of acute migraine (The TARGET Study)
- PMID: 25355310
- PMCID: PMC4320758
- DOI: 10.1111/head.12472
A randomized, double-blind, placebo-controlled study of breath powered nasal delivery of sumatriptan powder (AVP-825) in the treatment of acute migraine (The TARGET Study)
Abstract
Objective: To evaluate the efficacy and safety of AVP-825, a drug-device combination of low-dose sumatriptan powder (22 mg loaded dose) delivered intranasally through a targeted Breath Powered device vs an identical device containing lactose powder (placebo device) in the treatment of migraine headache.
Background: Early treatment of migraine headaches is associated with improved outcome, but medication absorption after oral delivery may be delayed in migraineurs because of reduced gastric motility. Sumatriptan powder administered with an innovative, closed-palate, Bi-Directional, Breath Powered intranasal delivery mechanism is efficiently absorbed across the nasal mucosa and produces fast absorption into the circulation. Results from a previously conducted placebo-controlled study of AVP-825 showed a high degree of headache relief with an early onset of action (eg, 74% AVP-825 vs 38% placebo device at 1 hour, P<.01).
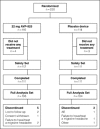
Methods: In this double-blind, placebo-controlled, parallel-group study in adults with a history of migraine with or without aura, participants were randomized via computer-generated lists to AVP-825 or placebo device to treat a single migraine headache of moderate or severe intensity. The primary endpoint was headache relief (defined as reduction of headache pain intensity from severe or moderate migraine headache to mild or none) at 2 hours post-dose.
Results: Two hundred and thirty patients (116 AVP-825 and 114 placebo device) were randomized, of whom 223 (112 and 111, respectively) experienced a qualifying migraine headache (their next migraine headache that reached moderate or severe intensity). A significantly greater proportion of AVP-825 patients reported headache relief at 2 hours post-dose compared with those using the placebo device (68% vs 45%, P=.002, odds ratio 2.53, 95% confidence interval [1.45, 4.42]). Between-group differences in headache relief were evident as early as 15 minutes, reached statistical significance at 30 minutes post-dose (42% vs 27%, P=.03), and were sustained at 24 hours (44% vs 24%, P=.002) and 48 hours (34% vs 20%, P=.01). Thirty-four percent of patients treated with AVP-825 were pain-free at 2 hours compared with 17% using the placebo device (P=.008). More AVP-825 patients reported meaningful pain relief (patient interpretation) of migraine within 2 hours of treatment vs placebo device (70% vs 45%, P<.001), and fewer required rescue medication (37% vs 52%, P=.02). Total migraine freedom (patients with no headache, nausea, phonophobia, photophobia, or vomiting) reached significance following treatment with AVP-825 at 1 hour (19% vs 9%; P=.04). There were no serious adverse events (AEs), and no systemic AEs occurred in more than one patient. Chest pain or pressure was not reported, and only one patient taking AVP-825 reported mild paresthesia. No other triptan sensations were reported.
Conclusions: Targeted delivery of a low-dose of sumatriptan powder via a novel, closed-palate, Breath Powered, intranasal device (AVP-825) provided fast relief of moderate or severe migraine headache in adults that reached statistical significance over placebo by 30 minutes. The treatment was well tolerated with a low incidence of systemic AEs.
Trial registration: ClinicalTrials.gov NCT01462812.
Keywords: AVP-825; Bi-Directional nasal delivery; Breath Powered nasal delivery; intranasal delivery; migraine; sumatriptan powder.
© 2014 The Authors. Headache published by Wiley Periodicals, Inc. on behalf of American Headache Society.
Figures

Similar articles
-
AVP-825 breath-powered intranasal delivery system containing 22 mg sumatriptan powder vs 100 mg oral sumatriptan in the acute treatment of migraines (The COMPASS study): a comparative randomized clinical trial across multiple attacks.Headache. 2015 May;55(5):621-35. doi: 10.1111/head.12583. Epub 2015 May 4. Headache. 2015. PMID: 25941016 Free PMC article. Clinical Trial.
-
Early Onset of Efficacy and Consistency of Response Across Multiple Migraine Attacks From the Randomized COMPASS Study: AVP-825 Breath Powered® Exhalation Delivery System (Sumatriptan Nasal Powder) vs Oral Sumatriptan.Headache. 2017 Jun;57(6):862-876. doi: 10.1111/head.13105. Epub 2017 May 11. Headache. 2017. PMID: 28497569 Clinical Trial.
-
DFN-02 (Sumatriptan 10 mg With a Permeation Enhancer) Nasal Spray vs Placebo in the Acute Treatment of Migraine: A Double-Blind, Placebo-Controlled Study.Headache. 2018 May;58(5):676-687. doi: 10.1111/head.13309. Headache. 2018. PMID: 29878341 Clinical Trial.
-
A review of clinical safety data for sumatriptan nasal powder administered by a breath powered exhalation delivery system in the acute treatment of migraine.Expert Opin Drug Saf. 2018 Jan;17(1):89-97. doi: 10.1080/14740338.2018.1390563. Epub 2017 Oct 20. Expert Opin Drug Saf. 2018. PMID: 28994319 Review.
-
A novel intranasal breath-powered delivery system for sumatriptan: a review of technology and clinical application of the investigational product AVP-825 in the treatment of migraine.Expert Opin Drug Deliv. 2015;12(9):1565-77. doi: 10.1517/17425247.2015.1060959. Epub 2015 Jun 29. Expert Opin Drug Deliv. 2015. PMID: 26119828 Review.
Cited by
-
A double-blind controlled clinical trial to evaluate the effects of nasal therapy with Vrihatajivakadya oil on different viscosities in patients with migraine.J Ayurveda Integr Med. 2023 Mar-Apr;14(2):100662. doi: 10.1016/j.jaim.2022.100662. Epub 2022 Nov 13. J Ayurveda Integr Med. 2023. PMID: 36384709 Free PMC article.
-
Reduction of pain and functional disability over time in patients treated with zavegepant: a post-hoc analysis of the BHV3500-301 phase 3 randomized controlled trial.J Headache Pain. 2025 Jan 2;26(1):1. doi: 10.1186/s10194-024-01915-y. J Headache Pain. 2025. PMID: 39748312 Free PMC article. Clinical Trial.
-
Nasal Delivery of Acute Medications for Migraine: The Upper Versus Lower Nasal Space.J Clin Med. 2021 Jun 2;10(11):2468. doi: 10.3390/jcm10112468. J Clin Med. 2021. PMID: 34199479 Free PMC article. Review.
-
Emerging treatments for the primary headache disorders.Neurol Sci. 2015 May;36 Suppl 1:109-13. doi: 10.1007/s10072-015-2133-1. Neurol Sci. 2015. PMID: 26017524 Review.
-
Effect of Ubrogepant vs Placebo on Pain and the Most Bothersome Associated Symptom in the Acute Treatment of Migraine: The ACHIEVE II Randomized Clinical Trial.JAMA. 2019 Nov 19;322(19):1887-1898. doi: 10.1001/jama.2019.16711. JAMA. 2019. PMID: 31742631 Free PMC article. Clinical Trial.
References
-
- Thorlund K, Mills EJ, Wu P. Comparative efficacy of triptans for the abortive treatment of migraine: A multiple treatment comparison meta-analysis. Cephalalgia. 2014;34:258–267. - PubMed
-
- Aurora SK, Kori SH, Barrodale P, McDonald SA, Haseley D. Gastric stasis in migraine: More than just a paroxysmal abnormality during a migraine attack. Headache. 2006;46:57–63. - PubMed
-
- Silberstein SD, Marcus DA. Sumatriptan: Treatment across the full spectrum of migraine. Expert Opin Pharmacother. 2013;14:1659–1667. - PubMed
-
- Rapoport A. New frontiers in headache therapy. Neurol Sci. 2011;32(Suppl. 1):S105–S109. - PubMed
-
- Rapoport AM. The therapeutic future in headache. Neurol Sci. 2012;33(Suppl. 1):S119–S125. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

