Icariin attenuates hypoxia-induced oxidative stress and apoptosis in osteoblasts and preserves their osteogenic differentiation potential in vitro
- PMID: 25355404
- PMCID: PMC6496789
- DOI: 10.1111/cpr.12147
Icariin attenuates hypoxia-induced oxidative stress and apoptosis in osteoblasts and preserves their osteogenic differentiation potential in vitro
Abstract
Objectives: Icariin, a prenylated flavonol glycoside isolated from traditional Chinese medicinal herb of the genus Epimedium, has been demonstrated to be a potential alternative therapy for osteoporosis, and its action mechanism so far has been mainly attributed to its phytoestrogenic property. As blood supply to bone is considerably reduced with ageing and by the menopause, we hypothesized that icariin treatment would reduce bone loss by preventing ischaemia-induced hypoxic damages to bone.
Materials and methods: To investigate effects of icariin treatment on cultured rat calvarial osteoblasts exposed to hypoxic conditions (2% oxygen).
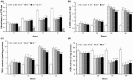
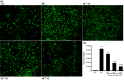
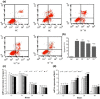
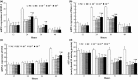
Results: Compared to normoxic control, cell viability decreased with time to 50% by 48 h in the hypoxic group, and icariin attenuated the reduction, dose dependently, with 10(-6) and 10(-5) m concentrations showing significant protective effects. Icariin also inhibited increase of lactate dehydrogenase activity in culture media. Measurements on oxidative stress, cell cycling and cell survival indicated that icariin protected osteoblasts by reducing production of reactive oxygen species and malondialdehyde, increasing superoxide dismutase activity, arresting the cell cycle and inhibiting apoptosis. Icariin also preserved osteogenic differentiation potential of the hypoxic cells in a dose-dependent manner, compared to the hypoxia alone group, as revealed by increased levels of RUNX-2, OSX and BMP-2 gene expression, alkaline phosphatase activity, and formation of mineralized nodules.
Conclusions: Our results demonstrated that icariin attenuated oxidative stress and apoptosis and preserved viability and osteogenic potential of osteoblasts exposed to hypoxia in vitro, and suggested that its anti-osteoporotic effect may be attributed to its anti-hypoxic activity and phytoestrogenic properties.
© 2014 John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
The effect of icariin on bone metabolism and its potential clinical application.Osteoporos Int. 2018 Mar;29(3):535-544. doi: 10.1007/s00198-017-4255-1. Epub 2017 Nov 6. Osteoporos Int. 2018. PMID: 29110063 Review.
-
Icariin is more potent than genistein in promoting osteoblast differentiation and mineralization in vitro.J Cell Biochem. 2011 Mar;112(3):916-23. doi: 10.1002/jcb.23007. J Cell Biochem. 2011. PMID: 21328465
-
The flavonol glycoside icariin promotes bone formation in growing rats by activating the cAMP signaling pathway in primary cilia of osteoblasts.J Biol Chem. 2017 Dec 22;292(51):20883-20896. doi: 10.1074/jbc.M117.809517. Epub 2017 Oct 31. J Biol Chem. 2017. PMID: 29089388 Free PMC article.
-
[Effects of icariin on the proliferation, differentiation and maturation of rat calvarial osteoblasts in vitro].Zhong Yao Cai. 2011 Jun;34(6):917-22. Zhong Yao Cai. 2011. PMID: 22017007 Chinese.
-
Functions and action mechanisms of flavonoids genistein and icariin in regulating bone remodeling.J Cell Physiol. 2013 Mar;228(3):513-21. doi: 10.1002/jcp.24158. J Cell Physiol. 2013. PMID: 22777826 Review.
Cited by
-
Flavonoids: Classification, Function, and Molecular Mechanisms Involved in Bone Remodelling.Front Endocrinol (Lausanne). 2021 Nov 23;12:779638. doi: 10.3389/fendo.2021.779638. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34887836 Free PMC article. Review.
-
Icariin has synergistic effects with methylprednisolone to ameliorate EAE via modulating HPA function, promoting anti-inflammatory and anti-apoptotic effects.Int J Clin Exp Med. 2015 Nov 15;8(11):20188-97. eCollection 2015. Int J Clin Exp Med. 2015. PMID: 26884931 Free PMC article.
-
Protective effects of icariin on cisplatin-induced acute renal injury in mice.Am J Transl Res. 2015 Oct 15;7(10):2105-14. eCollection 2015. Am J Transl Res. 2015. PMID: 26692955 Free PMC article.
-
Promoting osteogenesis and bone regeneration employing icariin-loaded nanoplatforms.J Biol Eng. 2024 Apr 22;18(1):29. doi: 10.1186/s13036-024-00425-4. J Biol Eng. 2024. PMID: 38649969 Free PMC article. Review.
-
The effect of icariin on bone metabolism and its potential clinical application.Osteoporos Int. 2018 Mar;29(3):535-544. doi: 10.1007/s00198-017-4255-1. Epub 2017 Nov 6. Osteoporos Int. 2018. PMID: 29110063 Review.
References
-
- Looker AC, Wahner HW, Dunn WL, Calvo MS, Harris TB, Heyse SP et al (1998) Updated data on proximal femur bone mineral levels of US adults. Osteoporos. Int. 8, 468–489. - PubMed
-
- Griffith JF, Kumta SM, Huang Y (2011) Hard arteries, weak bones. Skeletal Radiol. 40, 517–521. - PubMed
-
- London GM (2011) Soft bone – hard arteries: a link? Kidney Blood Press. Res. 34, 203–208. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

