New antibody approaches to lymphoma therapy
- PMID: 25355407
- PMCID: PMC4172963
- DOI: 10.1186/s13045-014-0058-4
New antibody approaches to lymphoma therapy
Abstract
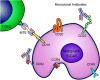
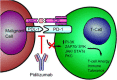
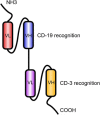
The CD20-directed monoclonal antibody rituximab established a new era in lymphoma therapy. Since then other epitopes on the lymphoma surface have been identified as potential targets for monoclonal antibodies (mAb). While most mAbs eliminate lymphoma cells mainly by antibody-dependent cellular cytotoxicity, complement-dependent cytotoxicity or direct cell death, others counter mechanisms utilized by malignant cells to evade immune surveillance. Expression of PD-L1 on malignant or stromal cells in the tumor environment for example leads to T-cell anergy. Targeting either PD-1 or PD-L1 via mAbs can indirectly eliminate cancer cells by unblocking the host intrinsic immune response. Yet another mechanism of targeted therapy with mAbs are bi-specific T-cell engagers (BiTE) such as blinatumomab, which directly engages the host immune cells. These examples highlight the broad spectrum of available therapies targeting the lymphoma surface with mAbs utilizing both passive and active immune pathways. Many of these agents have already demonstrated significant activity in clinical trials. In this review we will focus on novel CD20-directed antibodies as well as mAbs directed against newer targets like CD19, CD22, CD40, CD52 and CCR4. In addition we will review mAbs unblocking immune checkpoints and the BiTE blinatumomab. Given the success of mAbs and the expansion in active and passive immunotherapies, these agents will play an increasing role in the treatment of lymphomas.
Figures
References
-
- McLaughlin P, Grillo-Lopez AJ, Link BK, Levy R, Czuczman MS, Williams ME, Heyman MR, Bence-Bruckler I, White CA, Cabanillas F, Jain V, Ho AD, Lister J, Wey K, Shen D, Dallaire BK. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol. 1998;16:2825–2833. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

