Loss of α-catenin elicits a cholestatic response and impairs liver regeneration
- PMID: 25355493
- PMCID: PMC4213774
- DOI: 10.1038/srep06835
Loss of α-catenin elicits a cholestatic response and impairs liver regeneration
Abstract
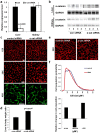
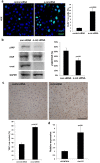
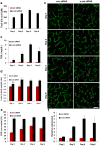
The liver is unique in its capacity to regenerate after injury, during which hepatocytes actively divide and establish cell-cell contacts through cell adhesion complexes. Here, we demonstrate that the loss of α-catenin, a well-established adhesion component, dramatically disrupts liver regeneration. Using a partial hepatectomy model, we show that regenerated livers from α-catenin knockdown mice are grossly larger than control regenerated livers, with an increase in cell size and proliferation. This increased proliferation correlated with increased YAP activation, implicating α-catenin in the Hippo/YAP pathway. Additionally, α-catenin knockdown mice exhibited a phenotype reminiscent of clinical cholestasis, with drastically altered bile canaliculi, elevated levels of bile components and signs of jaundice and inflammation. The disrupted regenerative capacity is a result of actin cytoskeletal disorganisation, leading to a loss of apical microvilli, dilated lumens in the bile canaliculi, and leaky tight junctions. This study illuminates a novel, essential role for α-catenin in liver regeneration.
Figures


Similar articles
-
YAP Activation Drives Liver Regeneration after Cholestatic Damage Induced by Rbpj Deletion.Int J Mol Sci. 2018 Nov 29;19(12):3801. doi: 10.3390/ijms19123801. Int J Mol Sci. 2018. PMID: 30501048 Free PMC article.
-
[Morphometric studies on the growth of bile canaliculi during liver regeneration in rats].Zhonghua Bing Li Xue Za Zhi. 1990 Dec;19(4):250-3. Zhonghua Bing Li Xue Za Zhi. 1990. PMID: 2081346 Chinese.
-
Liver regeneration requires Yap1-TGFβ-dependent epithelial-mesenchymal transition in hepatocytes.J Hepatol. 2018 Aug;69(2):359-367. doi: 10.1016/j.jhep.2018.05.008. Epub 2018 May 23. J Hepatol. 2018. PMID: 29758331 Free PMC article.
-
Bile canaliculi remodeling activates YAP via the actin cytoskeleton during liver regeneration.Mol Syst Biol. 2020 Feb;16(2):e8985. doi: 10.15252/msb.20198985. Mol Syst Biol. 2020. PMID: 32090478 Free PMC article.
-
Metallothionein and liver cell regeneration.Exp Biol Med (Maywood). 2006 Feb;231(2):138-44. doi: 10.1177/153537020623100203. Exp Biol Med (Maywood). 2006. PMID: 16446489 Review.
Cited by
-
Yes-associated protein impacts adherens junction assembly through regulating actin cytoskeleton organization.Am J Physiol Gastrointest Liver Physiol. 2016 Sep 1;311(3):G396-411. doi: 10.1152/ajpgi.00027.2016. Epub 2016 May 26. Am J Physiol Gastrointest Liver Physiol. 2016. PMID: 27229120 Free PMC article.
-
Loss of α(E)-catenin promotes Fas mediated apoptosis in tubular epithelial cells.Apoptosis. 2015 Jul;20(7):921-9. doi: 10.1007/s10495-015-1129-x. Apoptosis. 2015. PMID: 25894537 Free PMC article.
-
Extracellular matrix scaffolding guides lumen elongation by inducing anisotropic intercellular mechanical tension.Nat Cell Biol. 2016 Mar;18(3):311-8. doi: 10.1038/ncb3310. Epub 2016 Feb 15. Nat Cell Biol. 2016. PMID: 26878396
-
Cell Junctions in Hippo Signaling.Cold Spring Harb Perspect Biol. 2018 May 1;10(5):a028753. doi: 10.1101/cshperspect.a028753. Cold Spring Harb Perspect Biol. 2018. PMID: 28600393 Free PMC article. Review.
-
A Bile Duct-on-a-Chip With Organ-Level Functions.Hepatology. 2020 Apr;71(4):1350-1363. doi: 10.1002/hep.30918. Epub 2019 Oct 28. Hepatology. 2020. PMID: 31465556 Free PMC article.
References
-
- Foundation, A. L. Liver Wellness. Increasing Public Awareness of Liver Health, <http://www.liverfoundation.org/downloads/alf_download_29.pdf> (2009). Accessed 7th July 2014.
-
- Liver E. A. f. t. S. o. Facts and Figures. The Liver: Vital for Life, <http://old.easl.eu/eu_facts.asp> (2009). Accessed 7th July 2014.
-
- Mitchell C. & Willenbring H. A reproducible and well-tolerated method for 2/3 partial hepatectomy in mice. Nat. Protoc. 3, 1167–1170, 10.1038/nprot.2008.80 (2008). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

