A review of responsive MRI contrast agents: 2005-2014
- PMID: 25355685
- PMCID: PMC4414668
- DOI: 10.1002/cmmi.1629
A review of responsive MRI contrast agents: 2005-2014
Abstract
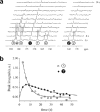
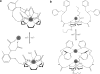
This review focuses on MRI contrast agents that are responsive to a change in a physiological biomarker. The response mechanisms are dependent on six physicochemical characteristics, including the accessibility of water to the agent, tumbling time, proton exchange rate, electron spin state, MR frequency or superparamagnetism of the agent. These characteristics can be affected by changes in concentrations or activities of enzymes, proteins, nucleic acids, metabolites, or metal ions, or changes in redox state, pH, temperature, or light. A total of 117 examples are presented, including ones that employ nuclei other than (1) H, which attests to the creativity of multidisciplinary research efforts to develop responsive MRI contrast agents.
Keywords: CEST agents; MRI contrast agents; MRS agents; T1 agents; T2* agents; hyperpolarized agents; responsive agents.
Copyright © 2014 John Wiley & Sons, Ltd.
Figures

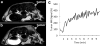









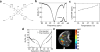
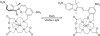
References
-
- Smith-Bindman R, Miglioretti DL, Johnson E, Lee C, Feigelson HS, Flynn M, Greenlee RT, Kruger RL, Hornbrook MC, Roblin D, Solberg LI, Vanneman N, Weinmann S, Williams AE. Use of diagnostic imaging studies and associated radiation exposure for patients enrolled in large integrated health care systems, 1996–2010. J Am Med Assoc. 2012;307(22):2400–2409. doi: 10.1001/jama.2012.5960. - DOI - PMC - PubMed
-
- Organisation for Economic Co-operation and Development. Health at a glance 2011: OECD indicators. OECD Publishing; Paris, France: 2011.
-
- Young IR, Clarke GJ, Bailes DR, Pennock JM, Doyle FH, Bydder GM. Enhancement of relaxation rate with paramagnetic contrast agents in NMR imaging. J Comput Tomogr. 1981;5(6):543–547. - PubMed
-
- Brady TJ, Goldman MR, Pykett IL, Buonanno FS, Kistler JP, Newhouse JH, Burt CT, Hinshaw WS, Pohost GM. Proton nuclear magnetic resonance imaging of regionally ischemic canine hearts: Effect of paramagnetic proton signal enhancement. Radiol. 1982;144(2):343–347. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
