Efficient and cost-effective generation of mature neurons from human induced pluripotent stem cells
- PMID: 25355730
- PMCID: PMC4250204
- DOI: 10.5966/sctm.2014-0024
Efficient and cost-effective generation of mature neurons from human induced pluripotent stem cells
Abstract
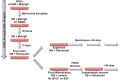
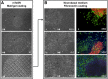
For years, our ability to study pathological changes in neurological diseases has been hampered by the lack of relevant models until the recent groundbreaking work from Yamanaka's group showing that it is feasible to generate induced pluripotent stem cells (iPSCs) from human somatic cells and to redirect the fate of these iPSCs into differentiated cells. In particular, much interest has focused on the ability to differentiate human iPSCs into neuronal progenitors and functional neurons for relevance to a large number of pathologies including mental retardation and behavioral or degenerative syndromes. Current differentiation protocols are time-consuming and generate limited amounts of cells, hindering use on a large scale. We describe a feeder-free method relying on the use of a chemically defined medium that overcomes the need for embryoid body formation and neuronal rosette isolation for neuronal precursors and terminally differentiated neuron production. Four days after induction, expression of markers of the neurectoderm lineage is detectable. Between 4 and 7 days, neuronal precursors can be expanded, frozen, and thawed without loss of proliferation and differentiation capacities or further differentiated. Terminal differentiation into the different subtypes of mature neurons found in the human brain were observed. At 6-35 days after induction, cells express typical voltage-gated and ionotrophic receptors for GABA, glycine, and acetylcholine. This specific and efficient single-step strategy in a chemically defined medium allows the production of mature neurons in 20-40 days with multiple applications, especially for modeling human pathologies.
Keywords: Dopaminergic neuron; GABA and glycine receptors; Human induced pluripotent cells; Neural differentiation; Neural induction; Neuronal progenitors; Patch clamp; Voltage-gated currents.
©AlphaMed Press.
Figures



References
-
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. - PubMed
-
- Park IH, Zhao R, West JA, et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–146. - PubMed
-
- Chamberlain SJ, Li XJ, Lalande M. Induced pluripotent stem (iPS) cells as in vitro models of human neurogenetic disorders. Neurogenetics. 2008;9:227–235. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

