Modulation of type I interferon-associated viral sensing during acute simian immunodeficiency virus infection in African green monkeys
- PMID: 25355871
- PMCID: PMC4301160
- DOI: 10.1128/JVI.02430-14
Modulation of type I interferon-associated viral sensing during acute simian immunodeficiency virus infection in African green monkeys
Abstract
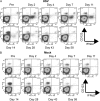
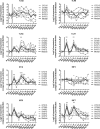
Natural hosts of simian immunodeficiency virus (SIV), such as African green monkeys (AGMs), do not progress to AIDS when infected with SIV. This is associated with an absence of a chronic type I interferon (IFN-I) signature. It is unclear how the IFN-I response is downmodulated in AGMs. We longitudinally assessed the capacity of AGM blood cells to produce IFN-I in response to SIV and herpes simplex virus (HSV) infection. Phenotypes and functions of plasmacytoid dendritic cells (pDCs) and other mononuclear blood cells were assessed by flow cytometry, and expression of viral sensors was measured by reverse transcription-PCR. pDCs displayed low BDCA-2, CD40, and HLA-DR expression levels during AGM acute SIV (SIVagm) infection. BDCA-2 was required for sensing of SIV, but not of HSV, by pDCs. In acute infection, AGM peripheral blood mononuclear cells (PBMCs) produced less IFN-I upon SIV stimulation. In the chronic phase, the production was normal, confirming that the lack of chronic inflammation is not due to a sensing defect of pDCs. In contrast to stimulation by SIV, more IFN-I was produced upon HSV stimulation of PBMCs isolated during acute infection, while the frequency of AGM pDCs producing IFN-I upon in vitro stimulation with HSV was diminished. Indeed, other cells started producing IFN-I. This increased viral sensing by non-pDCs was associated with an upregulation of Toll-like receptor 3 and IFN-γ-inducible protein 16 caused by IFN-I in acute SIVagm infection. Our results suggest that, as in pathogenic SIVmac infection, SIVagm infection mobilizes bone marrow precursor pDCs. Moreover, we show that SIV infection modifies the capacity of viral sensing in cells other than pDCs, which could drive IFN-I production in specific settings.
Importance: The effects of HIV/SIV infections on the capacity of plasmacytoid dendritic cells (pDCs) to produce IFN-I in vivo are still incompletely defined. As IFN-I can restrict viral replication, contribute to inflammation, and influence immune responses, alteration of this capacity could impact the viral reservoir size. We observed that even in nonpathogenic SIV infection, the frequency of pDCs capable of efficiently sensing SIV and producing IFN-I was reduced during acute infection. We discovered that, concomitantly, cells other than pDCs had increased abilities for viral sensing. Our results suggest that pDC-produced IFN-I upregulates viral sensors in bystander cells, the latter gaining the capacity to produce IFN-I. These results indicate that in certain settings, cells other than pDCs can drive IFN-I-associated inflammation in SIV infection. This has important implications for the understanding of persistent inflammation and the establishment of viral reservoirs.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures




References
-
- Fonteneau J-F, Larsson M, Beignon A-S, McKenna K, Dasilva I, Amara A, Liu Y-J, Lifson JD, Littman DR, Bhardwaj N. 2004. Human immunodeficiency virus type 1 activates plasmacytoid dendritic cells and concomitantly induces the bystander maturation of myeloid dendritic cells. J Virol 78:5223–5232. doi: 10.1128/JVI.78.10.5223-5232.2004. - DOI - PMC - PubMed
-
- Romagnani C, Della Chiesa M, Kohler S, Moewes B, Radbruch A, Moretta L, Moretta A, Thiel A. 2005. Activation of human NK cells by plasmacytoid dendritic cells and its modulation by CD4+ T helper cells and CD4+ CD25hi T regulatory cells. Eur J Immunol 35:2452–2458. doi: 10.1002/eji.200526069. - DOI - PubMed
-
- Karpov AV. 2001. Endogenous and exogenous interferons in HIV-infection. Eur J Med Res 6:507–524. - PubMed
-
- Asmuth DM, Murphy RL, Rosenkranz SL, Lertora JJ, Kottilil S, Cramer Y, Chan ES, Schooley RT, Rinaldo CR, Thielman N, Li XD, Wahl SM, Shore J, Janik J, Lempicki RA, Simpson Y, Pollard RB. 2010. Safety, tolerability, and mechanisms of antiretroviral activity of pegylated interferon Alfa-2a in HIV-1-monoinfected participants: a phase II clinical trial. J Infect Dis 201:1686–1696. doi: 10.1086/652420. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

