A newly isolated reovirus has the simplest genomic and structural organization of any reovirus
- PMID: 25355879
- PMCID: PMC4301156
- DOI: 10.1128/JVI.02264-14
A newly isolated reovirus has the simplest genomic and structural organization of any reovirus
Abstract
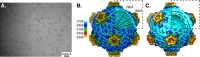
A total of 2,691 mosquitoes representing 17 species was collected from eight locations in southwest Cameroon and screened for pathogenic viruses. Ten isolates of a novel reovirus (genus Dinovernavirus) were detected by culturing mosquito pools on Aedes albopictus (C6/36) cell cultures. A virus that caused overt cytopathic effects was isolated, but it did not infect vertebrate cells or produce detectable disease in infant mice after intracerebral inoculation. The virus, tentatively designated Fako virus (FAKV), represents the first 9-segment, double-stranded RNA (dsRNA) virus to be isolated in nature. FAKV appears to have a broad mosquito host range, and its detection in male specimens suggests mosquito-to-mosquito transmission in nature. The structure of the T=1 FAKV virion, determined to subnanometer resolution by cryoelectron microscopy (cryo-EM), showed only four proteins per icosahedral asymmetric unit: a dimer of the major capsid protein, one turret protein, and one clamp protein. While all other turreted reoviruses of known structures have at least two copies of the clamp protein per asymmetric unit, FAKV's clamp protein bound at only one conformer of the major capsid protein. The FAKV capsid architecture and genome organization represent the most simplified reovirus described to date, and phylogenetic analysis suggests that it arose from a more complex ancestor by serial loss-of-function events.
Importance: We describe the detection, genetic, phenotypic, and structural characteristics of a novel Dinovernavirus species isolated from mosquitoes collected in Cameroon. The virus, tentatively designated Fako virus (FAKV), is related to both single-shelled and partially double-shelled viruses. The only other described virus in this genus was isolated from cultured mosquito cells. It was previously unclear whether the phenotypic characteristics of that virus were reflective of this genus in nature or were altered during serial passaging in the chronically infected cell line. FAKV is a naturally occurring single-shelled reovirus with a unique virion architecture that lacks several key structural elements thought to stabilize a single-shelled reovirus virion, suggesting what may be the minimal number of proteins needed to form a viable reovirus particle. FAKV evolved from more complex ancestors by losing a genome segment and several virion proteins.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures




References
-
- Attoui H, Mertens PPC, Becnel J, Belaganahalli S, Bergoin M, Brussard CP, Chappell Ciarlet JDM, del Vas M, Dermody TS, Dormitzer PR, Duncan R, Fcang Q, Graham R, Guglielmi KM, Harding RM, Hillman B, Makkay A, Marzachi C, Matthijnssens J, Milne RG, Mohd Jaafar F, Mori H, Noordeloos AA, Omura T, Patton JT, Rao S, Maan M, Stoltz D, Suzuki N, Upadhyaya NM, Wei C, Zhou H. 2012. Family Reoviridae, p 541–637. In King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ (ed), Virus taxonomy: ninth report of the International Committee on Taxonomy of Viruses. Academic Press, London, United Kingdom.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

