Activity-dependent PI(3,5)P2 synthesis controls AMPA receptor trafficking during synaptic depression
- PMID: 25355904
- PMCID: PMC4234577
- DOI: 10.1073/pnas.1411117111
Activity-dependent PI(3,5)P2 synthesis controls AMPA receptor trafficking during synaptic depression
Abstract
Dynamic regulation of phosphoinositide lipids (PIPs) is crucial for diverse cellular functions, and, in neurons, PIPs regulate membrane trafficking events that control synapse function. Neurons are particularly sensitive to the levels of the low abundant PIP, phosphatidylinositol 3,5-bisphosphate [PI(3,5)P2], because mutations in PI(3,5)P2-related genes are implicated in multiple neurological disorders, including epilepsy, severe neuropathy, and neurodegeneration. Despite the importance of PI(3,5)P2 for neural function, surprisingly little is known about this signaling lipid in neurons, or any cell type. Notably, the mammalian homolog of yeast vacuole segregation mutant (Vac14), a scaffold for the PI(3,5)P2 synthesis complex, is concentrated at excitatory synapses, suggesting a potential role for PI(3,5)P2 in controlling synapse function and/or plasticity. PI(3,5)P2 is generated from phosphatidylinositol 3-phosphate (PI3P) by the lipid kinase PI3P 5-kinase (PIKfyve). Here, we present methods to measure and control PI(3,5)P2 synthesis in hippocampal neurons and show that changes in neural activity dynamically regulate the levels of multiple PIPs, with PI(3,5)P2 being among the most dynamic. The levels of PI(3,5)P2 in neurons increased during two distinct forms of synaptic depression, and inhibition of PIKfyve activity prevented or reversed induction of synaptic weakening. Moreover, altering neuronal PI(3,5)P2 levels was sufficient to regulate synaptic strength bidirectionally, with enhanced synaptic function accompanying loss of PI(3,5)P2 and reduced synaptic strength following increased PI(3,5)P2 levels. Finally, inhibiting PI(3,5)P2 synthesis alters endocytosis and recycling of AMPA-type glutamate receptors (AMPARs), implicating PI(3,5)P2 dynamics in AMPAR trafficking. Together, these data identify PI(3,5)P2-dependent signaling as a regulatory pathway that is critical for activity-dependent changes in synapse strength.
Keywords: Fab1; PIKfyve; Vac14; phosphatidylinositol lipids; synaptic plasticity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


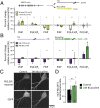
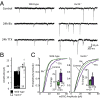



References
-
- Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443(7112):651–657. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

