CD28-CD80 interactions control regulatory T cell motility and immunological synapse formation
- PMID: 25355918
- PMCID: PMC4258405
- DOI: 10.4049/jimmunol.1401752
CD28-CD80 interactions control regulatory T cell motility and immunological synapse formation
Abstract
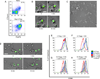
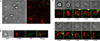
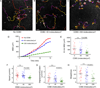
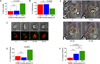
Regulatory T cells (Tregs) are essential for tolerance to self and environmental Ags, acting in part by downmodulating costimulatory molecules on the surface of dendritic cells (DCs) and altering naive CD4 T cell-DC interactions. In this study, we show that Tregs form stable conjugates with DCs before, but not after, they decrease surface expression of the costimulatory molecule CD80 on the DCs. We use supported planar bilayers to show that Tregs dramatically slow down but maintain a highly polarized and motile phenotype after recognizing Ag in the absence of costimulation. These motile cells are characterized by distinct accumulations of LFA-1-ICAM-1 in the lamella and TCR-MHC in the uropod, consistent with a motile immunological synapse or "kinapse." However, in the presence of high, but not low, concentrations of CD80, Tregs form stationary, symmetrical synapses. Using blocking Abs, we show that, whereas CTLA-4 is required for CD80 downmodulation, CD28-CD80 interactions are critical for modulating Treg motility in the presence of Ag. Taken together, these results support the hypothesis that Tregs are tuned to alter their motility depending on costimulatory signals.
Copyright © 2014 by The American Association of Immunologists, Inc.
Figures



References
-
- Belkaid Y, Tarbell K. Regulatory T cells in the control of host-microorganism interactions. Annu Rev Immunol. 2009;27:551–589. - PubMed
-
- Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. - PubMed
-
- Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4(+)CD25(+) regulatory T cells. Nat Immunol. 2003;4:330–336. - PubMed
-
- Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337–342. - PubMed
-
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor foxp3. Science. 2003;299:1057–1061. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

