The Trabecular Meshwork: A Basic Review of Form and Function
- PMID: 25356439
- PMCID: PMC4209746
- DOI: 10.13188/2334-2838.1000017
The Trabecular Meshwork: A Basic Review of Form and Function
Figures



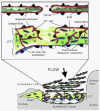
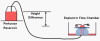
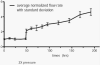
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
