Loss of basal and TRH-stimulated Tshb expression in dispersed pituitary cells
- PMID: 25356823
- PMCID: PMC4272397
- DOI: 10.1210/en.2014-1281
Loss of basal and TRH-stimulated Tshb expression in dispersed pituitary cells
Abstract
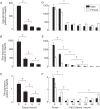
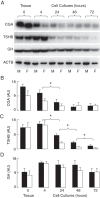
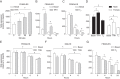
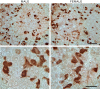
This study addresses the in vivo and in vitro expression pattern of three genes that are operative in the thyrotroph subpopulation of anterior pituitary cells: glycoprotein α-chain (Cga), thyroid-stimulating hormone β-chain (Tshb), and TRH receptor (Trhr). In vivo, the expression of Cga and Tshb was robust, whereas the expression of Trhr was low. In cultured pituitary cells, there was a progressive decline in the expression of Cga, Tshb, and Trhr. The expression of Tshb could not be reversed via pulsatile or continuous TRH application in variable concentrations and treatment duration or by the removal of thyroid and steroid hormones from the sera. In parallel, the expression of CGA and TSHB proteins declined progressively in pituitary cells from both sexes. The lack of the effect of TRH on Tshb expression was not related to the age of pituitary cultures and the presence of functional TRH receptors. In cultured pituitary fragments, there was also a rapid decline in expression of these genes, but TRH was able to induce transient Tshb expression. In vivo, thyrotrophs were often in close proximity to each other and to somatotroph and folliculostellate cell networks and especially to the lactotroph cell network; such an organization pattern was lost in vitro. These observations suggest that the lack of influence of anterior pituitary architecture and/or intrapituitary factors probably accounts for the loss of basal and TRH-stimulated Tshb expression in dispersed pituitary cells.
Figures



References
-
- Hodson DJ, Romano N, Schaeffer M, et al. Coordination of calcium signals by pituitary endocrine cells in situ. Cell Calcium. 2012;51(3–4):222–230. - PubMed
-
- Le Tissier PR, Hodson DJ, Lafont C, Fontanaud P, Schaeffer M, Mollard P. Anterior pituitary cell networks. Front Neuroendocrinol. 2012;33(3):252–266. - PubMed
-
- Mollard P, Hodson DJ, Lafont C, Rizzoti K, Drouin J. A tridimensional view of pituitary development and function. Trends Endocrinol Metab. 2012;23(6):261–269. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

